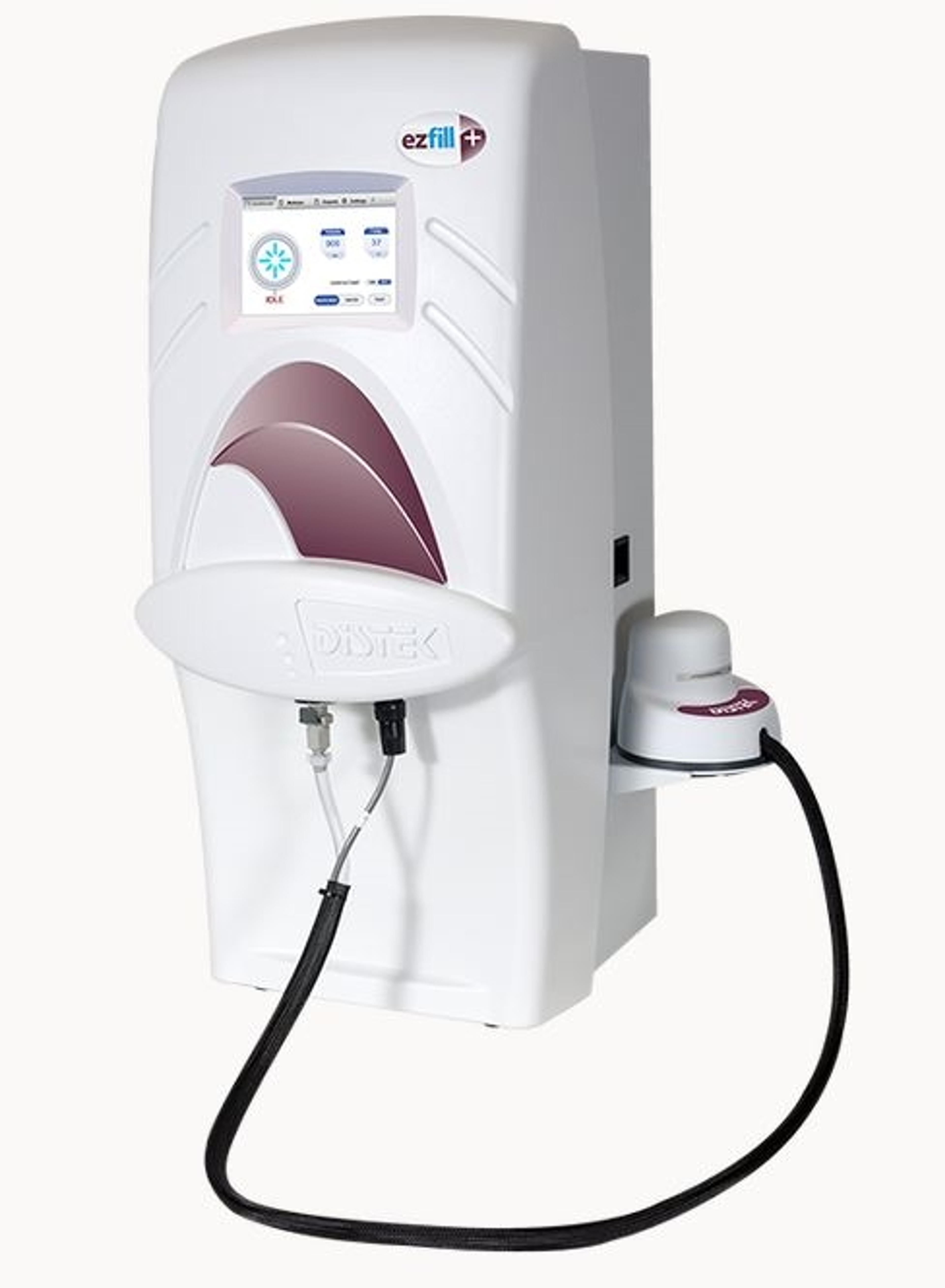
Model 2500 Water Bath Dissolution Instrument
Distek, Inc.The Distek Model 2500 Dissolution System offers great flexibility and configurability. It can be configured as USP Apparatus 1, 2, 5, and 6 plus intrinsic dissolution. The extensive users, method, and reporting storage capabilities are enabled by a brilliant touch screen.