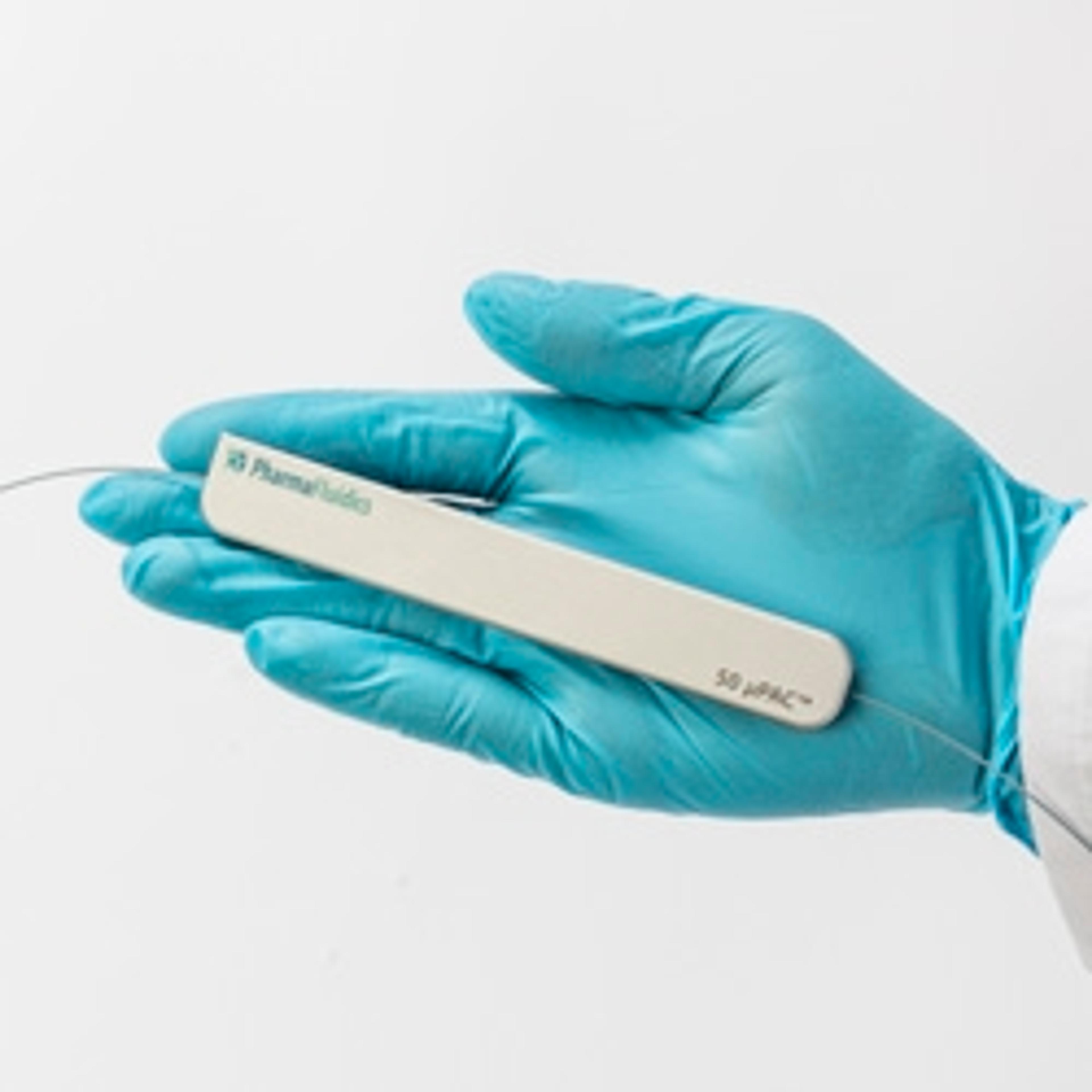
50 cm µPAC™ column
PharmaFluidicsWith an internal volume of 3µL, this 50 cm long RP-C18 µPAC™ column is perfectly suited to perform high throughput analyses with shorter gradient solvent times (30, 60 and 90-minute gradients) and it can be used over a wide range of flow rates, between 100 and 2000 nL/min. µPAC™ column, 50 cm length, with pillar array backbone at interpillar distance of 2.5µm, C18.