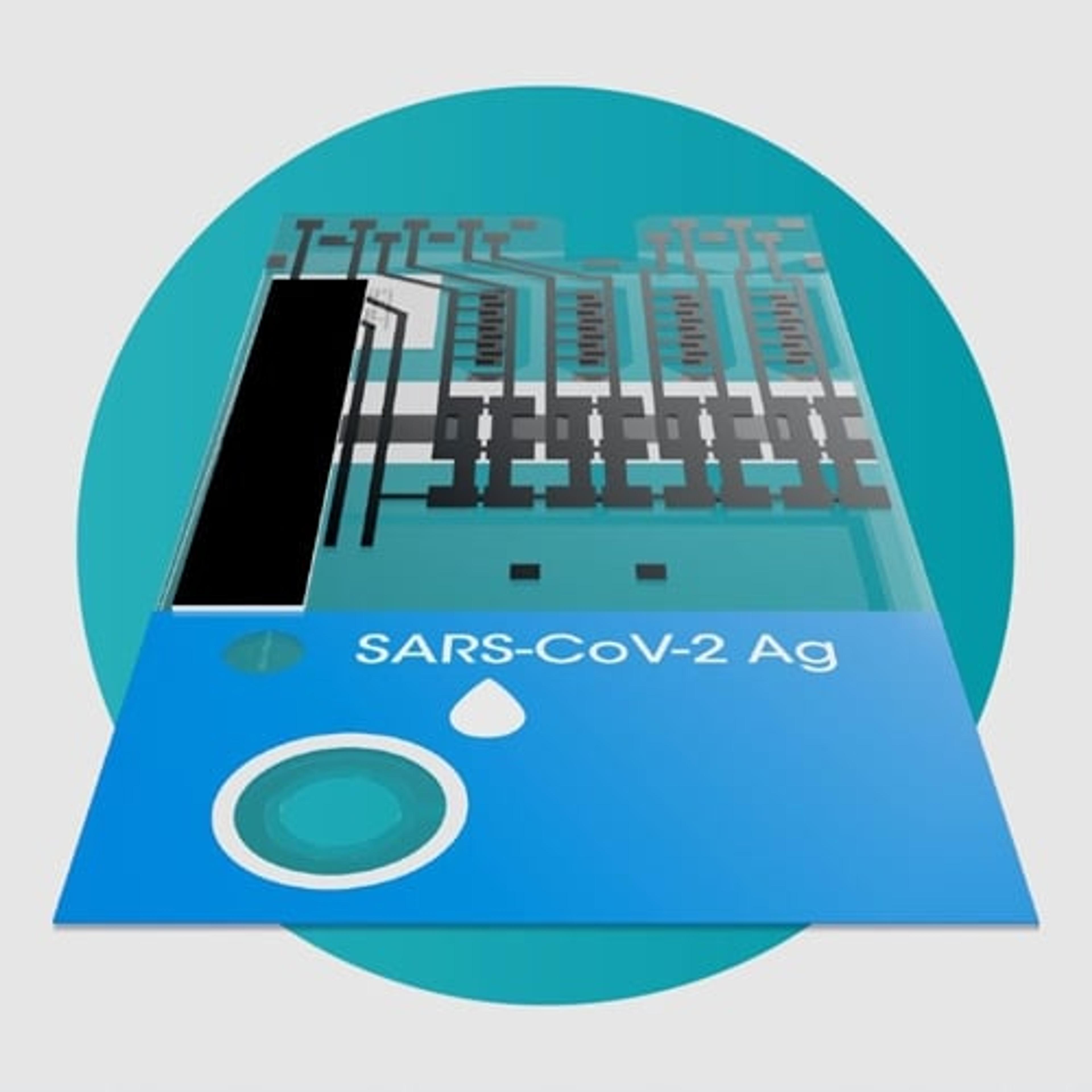
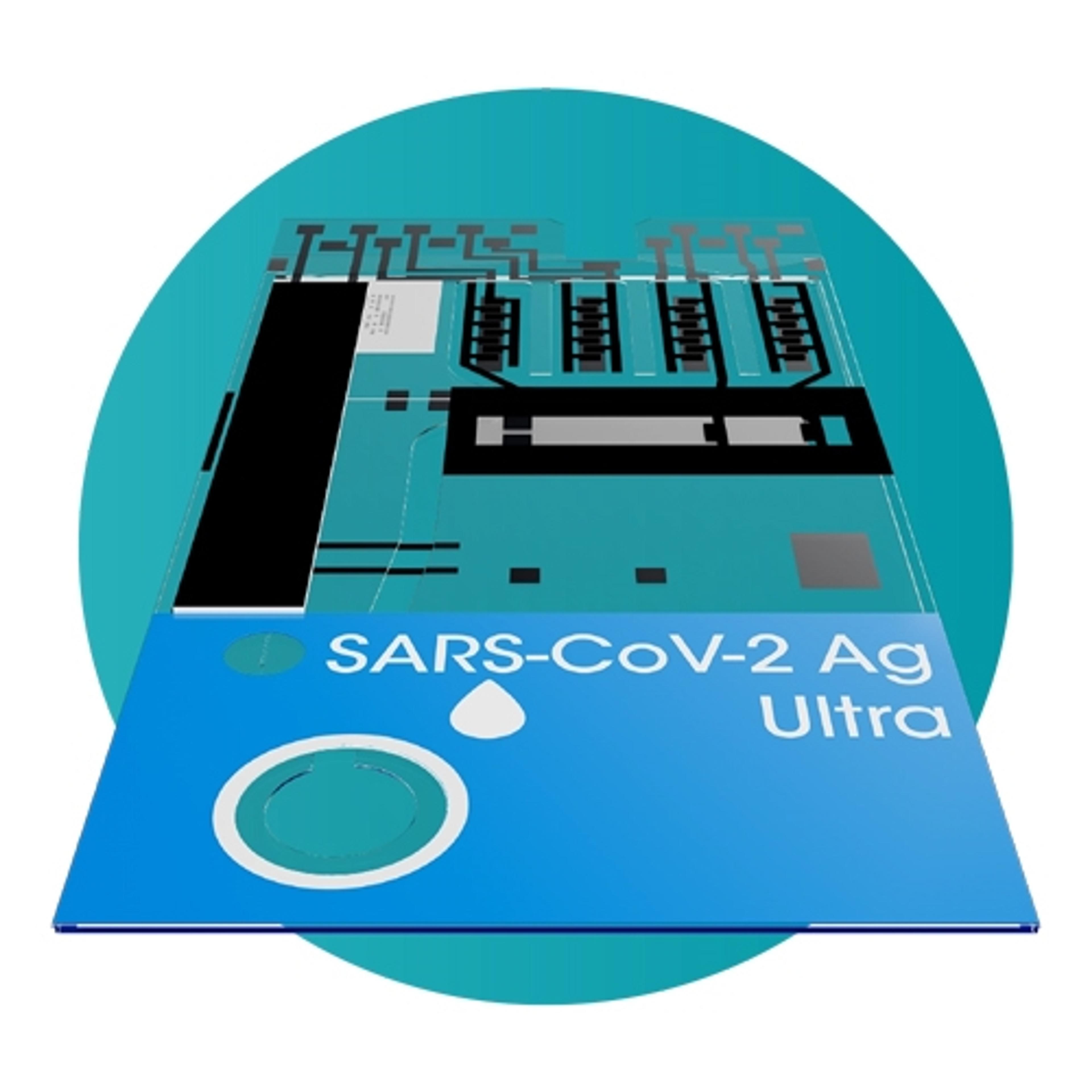
KeyPro™ KP100 UV LED Decontamination System
Phoseon TechnologyUV LED decontamination using Phoseon’s KeyPro™ KP100 instrument benefits labs that require high fidelity RNA library prep, sequencing and PCR, or for vaccine development labs that need to quickly and reliably inactivate viruses.