ResourceLife Sciences
Multi-Fluorescence Imaging of Protein Crystals
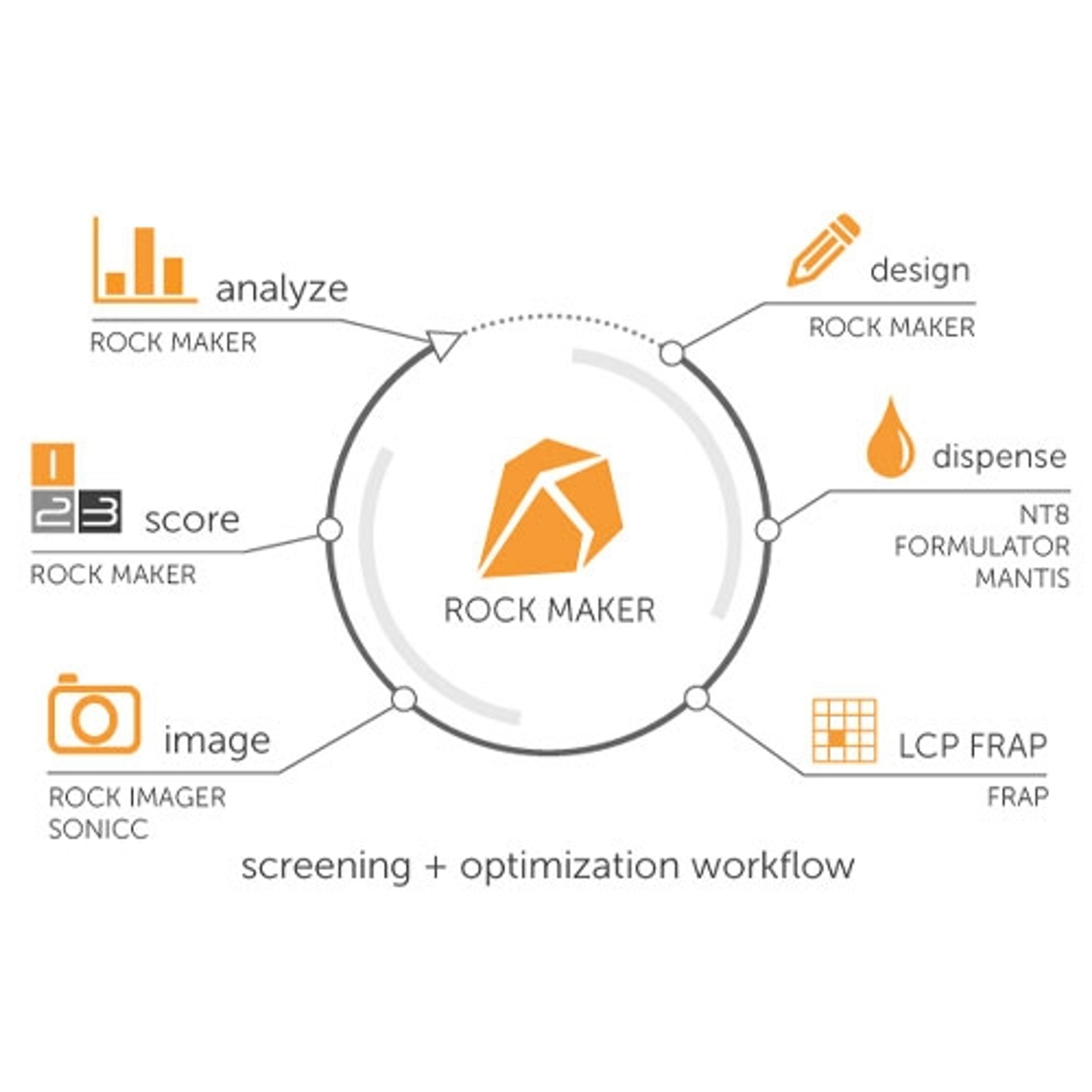
11 May 2017The unambiguous and reliable identification of biological crystals remains a major obstacle in crystallography, particularly in the critical stage of initial screening experiments. Crystallographers have often relied on the intrinsic fluorescence of aromatic amino acids, such as tryptophan, to differentiate between salt and protein crystals. In some cases, proteins have very few tryptophan amino acids, or none at all, making it impossible to use UV fluorescence for imaging. The addition of a fluorescent molecule to one’s protein enables the ability to do visible fluorescence imaging and acquire high contrast images in very short imaging times with no sample damage.