Search results for "radleys"
Selected Filters:
Enhancing efficiency and ease in quality control of wine
Delivery of genome editing tools to knockout target genes
INTACT Software Library product and services
Can I freeze a Samco Clicktainer Vial?
Abbott receives FDA clearance for its imaging technology using artificial intelligence for vessels in the heart
The new intuitive interface provides step-by-step guidance to enhance ease of use
Monitor multiple stages of apoptosis with live cell kinetic imaging
'Reviews get down to the reality of a product’s performance’
Lab manager Marisa Hildebrandt emphasizes the need for better communication in science and honest product reviews that ‘get past the hype’
Investigating SARS-CoV-2 variants of concern using single-well multiplex qPCR
Watch this on-demand webinar to gain insights into the design and development pipeline for rapid response solutions
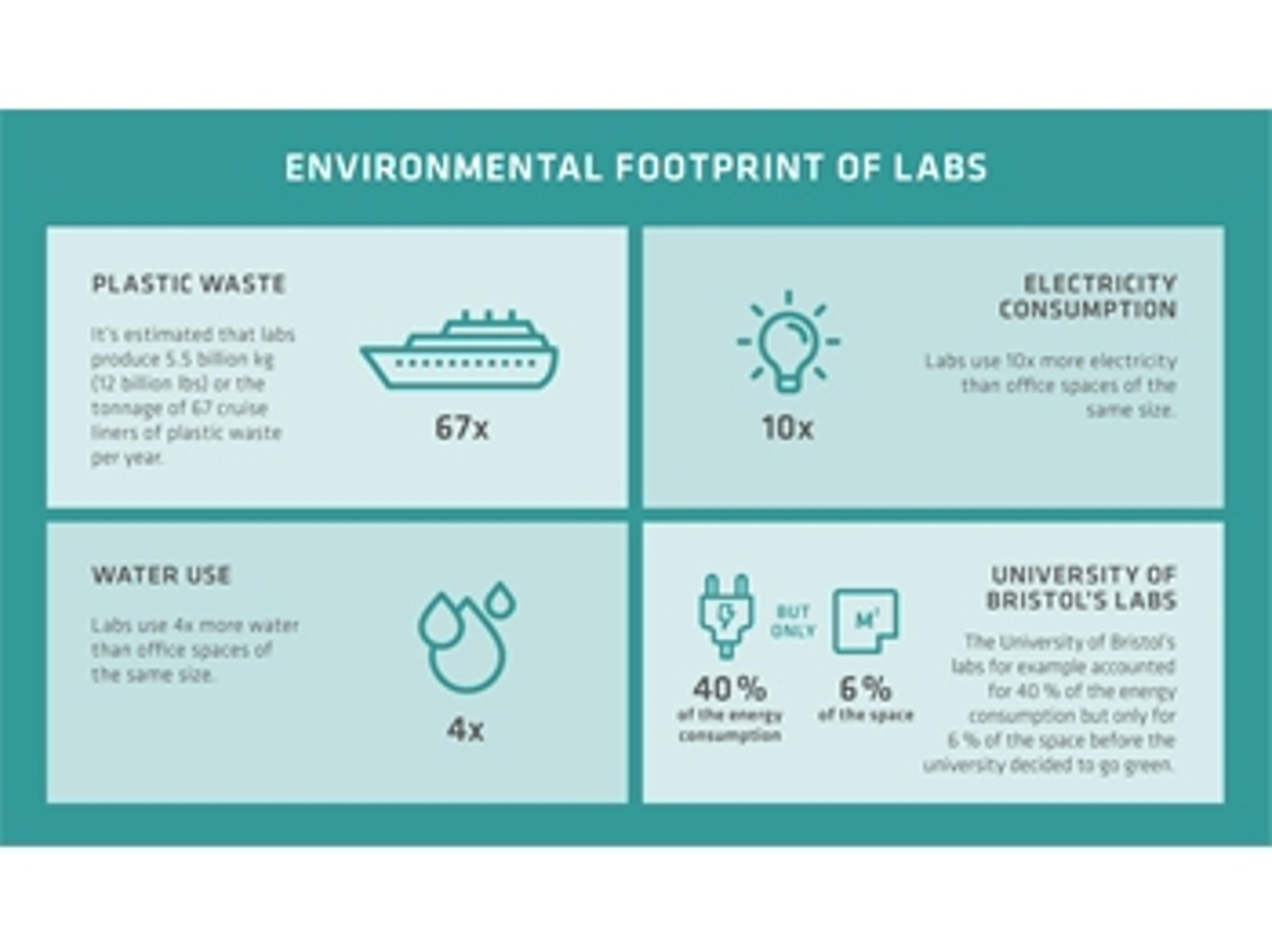
INTEGRA’s commitment to developing greener labs
INTEGRA Biosciences has created a list of key steps to control laboratory resource consumption and waste production in an attempt to reduce environmental footprint
Thermo Fisher Scientific collaborates with ChromSword to deliver rapid automated HPLC and UHPLC method development system
The new Thermo Scientific Vanquish Method Development HPLC and UHPLC system delivers robust and validated methods in less time with higher confidence
HORIBA to showcase novel POCKIT Central PCR analyzer at BEVA and Vets North
Vets can see for themselves how easy it is to do in-house PCR testing using POCKIT Central PCR analyzer
Digital obsolescence: Is your research data at risk?
Experts discuss how technological advances result in a ‘graveyard of software’ and describe a compliant solution designed to safeguard vital data for generations to come
7 upcoming webinars to take your research to the next level
Gain insights on topics from CRISPR and gene editing to biomarkers, liquid chromatography advancements and high-sensitivity POC cardiac troponin
Off-load gene therapy and vaccine buffer exchange to Big Tuna
Watch this on-demand webinar to learn about new methods tailored to each gene therapy vector type
Nobelpharma signs a license agreement for ProBioGen’s vaccine manufacturing platform AGE1.CR.pIX
Nobelpharma will use ProBioGen’s cell-based production platform AGE1.CR.pIX to develop their lead vaccine
Wastewater testing labs can detect SARS-CoV-2 in less than four hours with new methods
A complete workflow developed by Promega creates a snapshot of community-wide infection rates using wastewater samples