Data Agility in Biopharma - Strategies for Success
In the rapidly evolving landscape of biopharmaceutical product development, effective data strategies are essential for driving innovation, ensuring regulatory compliance, facilitating efficient decision-making, improving research outcomes, and accelerating development timelines.
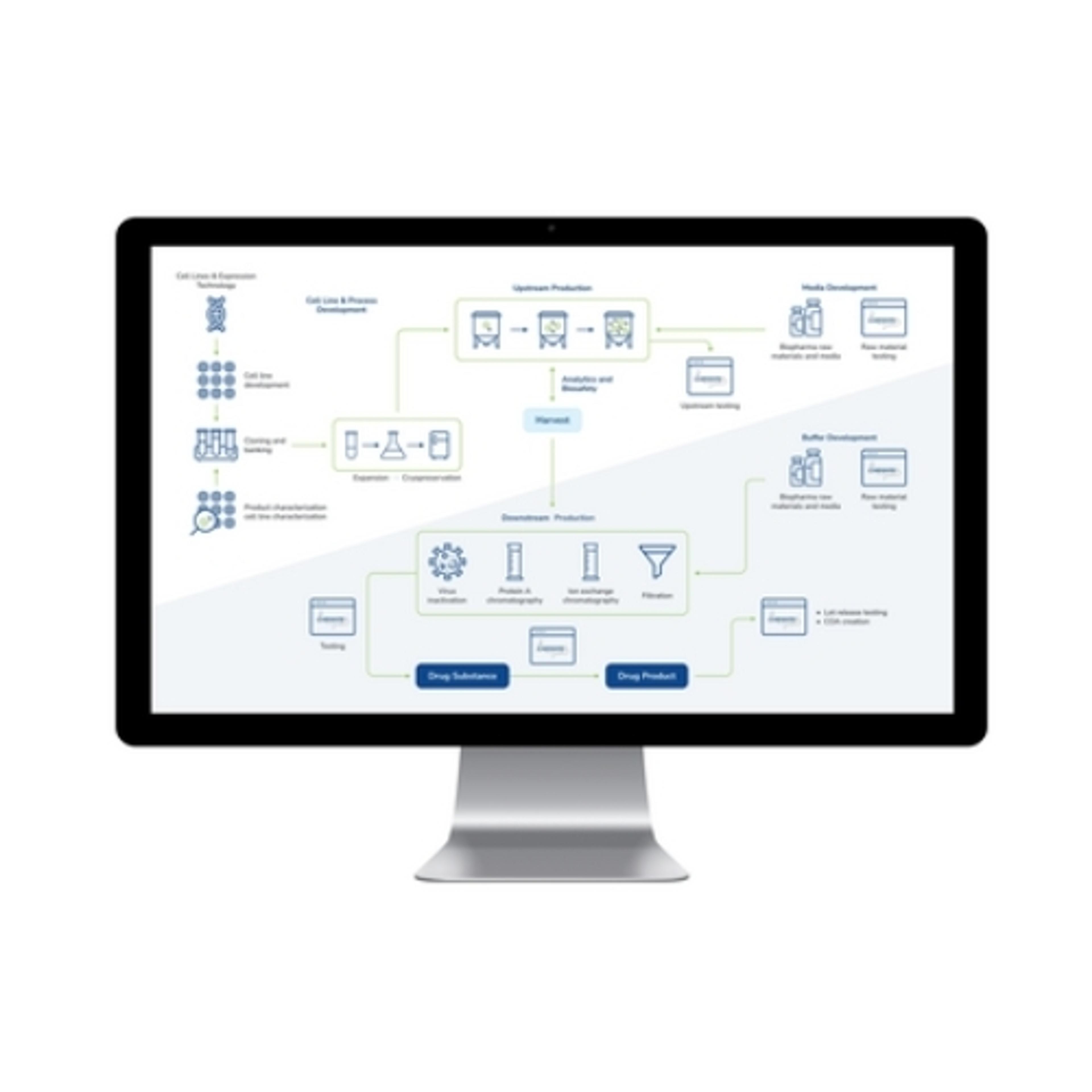
In this webinar, Tom Brohan, BioPharma Leader at LabWare, will detail the common and distinctive challenges encountered in biopharmaceutical laboratories and will provide practical strategies for overcoming them. Topics he will cover include data integration, volume, and complexity; data quality and standardization; data security and compliance; and access and collaboration challenges. Furthermore, the webinar will highlight the specific data management challenges faced by R&D, bioanalysis, and QA/QC bioprocessing laboratories, offering solutions to address these intricacies.
Key learning objectives
- Explore the common data challenges scientists face in biopharmaceutical research and development
- Discover practical strategies on how to overcome specific data challenges
- Learn new ways to unlock and interpret information from different data sources
Who should attend?
- Quality managers in biopharma operations
- R&D scientists in biopharma
- Lab managers
- Bioprocess engineers
Certificate of attendance
All webinar participants can request a certificate of attendance, including a learning outcomes summary, for continuing education purposes.
Speakers