Confidence in your knock-outs: The importance of robustly validated cell lines
Quality control and antibody validation expert Julie Wickenden shares how she works to ensure full protein knock-out in Abcam’s cell line portfolio
5 Jul 2021

In this exclusive SelectScience interview, we speak with Dr. Julie Wickenden, the Head of the Cell Sciences team for the New Product Development (NPD) pipeline at Abcam. Here, Wickenden shares insights into Abcam’s work to expand its current catalog and increase the NPD pipeline, an endeavor that aims to provide a comprehensive target offering that inspires confidence in its users.
What are the overall goals of your team at Abcam?
JW: My team has two functions. The first is involved in the quality control (QC) pipeline and provision of QC material. All of our antibodies are validated using cell lines, cell line derivatives, or cell-based models, and my team is responsible for growing these cell lines and performing compound treatments to enable the antibodies to be validated properly.
My team is also involved in producing and validating the stock of the cell lines, knock-outs, and lysates for sale in our catalog. Cell editing takes place at our site in Fremont, California, and the resulting KO cells are then sent to us for expansion, banking, and preparation for QC by the antibody characterization team.
What challenges are your customers currently facing and how does the Abcam offering aim to help with these?
JW: One of the main challenges that our customers have is ensuring that the antibodies are specific. Abcam is known for its antibodies, and we go a long way to prove that the antibodies that we produce are specific for the target that they're intended for. We do this validation using knock-out cell lines, as well as upregulation treatments, overexpression, siRNA, etc. When it comes to our cell line offering, people need to be sure that they have the correct controls and that things are working properly, and our knock-out cell lines are perfect for this. These are designed to help customers phenotypically interrogate their research hypotheses. Not only that, but they help with full pathway validation. So, when a user is doing their experiments including one of our knock-out cells, the user can be sure that the results that they're seeing are down to the lack of that protein.
How do you validate your cell lines?
JW: Our knock-out cell lines are validated genomically. We do next-generation sequencing (NGS) to ensure that we have a frameshift mutation in our gene. Unfortunately, just having a genomic knock-out doesn't guarantee that it's a protein knock-out, so we go one step further and perform proteomic validation by antibody-based technology. At the moment, this is mainly western blot, but if the target needs a different validation technique, then we will perform it. For example, with secreted cytokines, checking for the knock-out by western blot isn't appropriate and we would instead use ELISA.
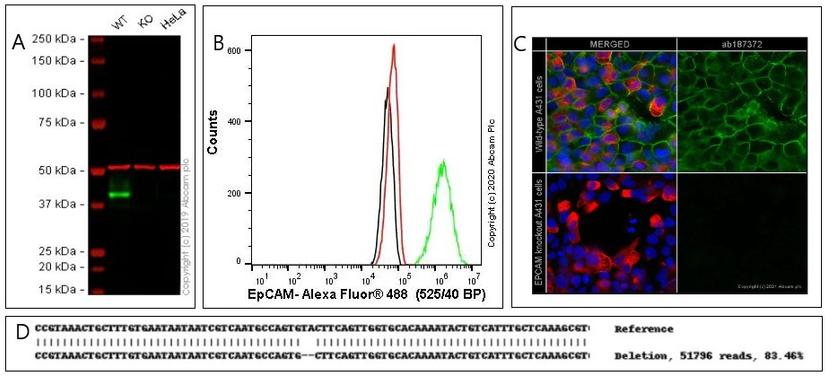
Why is having validated cell lines so important?
JW: Genes are more complex than we think. You could knock out a gene and not end up with knock-out of the protein. So, validation is an invaluable process to ensure there is no wasted money, time, or resources, and scientific credibility is not damaged. We do see this with some of our cell lines when we try to knock out certain genes. In this situation, we go back to one of our other clones, with a different edit, and we will check that as well. We do a lot of the work for our customers in terms of screening clones to make sure that they are indeed full knock-outs.
How is Abcam working to expand its current catalog and increase the pipeline?
JW: The plan is to expand our portfolio by the breadth of the gene knock-outs and protein knock-outs that we offer, as well as the backgrounds that we offer, in order to cover targets in key disease-relevant research areas such as neurodegeneration and particular types of cancer. To achieve this, we're using some of the latest technologies that have been used in higher-throughput CRISPR screening. In doing this, we’re hoping to improve availability of the targets and have as many targets as possible to cover the whole proteome.
In addition to the availability, the expansion is also aiming to better help with target-relevant models. For example, if we have a target that plays a role in Burkitt's lymphoma, we use a lymphoma-based cell line so that not only do you get the knock-out but you also ensure that all the other pathways in the cell behave as they should.
Aside from your current expansion, how is Abcam aiming to help researchers succeed in the future?
JW: Abcam works to offer the flexibility, expertise, and support researchers need, whatever their application. We have a custom pipeline, covering knock-ins to more complex CRISPR applications, along with additional assay services to ensure any and all cell engineering needs are met. If there is something you want to do, just come and talk to us.
Hear more from the knock-out experts:
- How to achieve research success with knock-out models – Dr. Hanna Dreja
- Speeding the transition from bench to bedside with CRISPR/Cas9 – Dr. Karine Enesa
- Breakthroughs in guide RNA: Creating successful CRISPR-Cas9 knock-outs – Dr. Yongwon Kwon
Find out more about five pillars to determine antibody specificity >>

