CRISPR Screening for Genome-Wide Investigation of Gene Function
Functional genomic screening using CRISPR-Cas9 gene editing technology for drug discovery, development and more
23 Jan 2019
In this interview, Dr. James Goldmeyer, product manager at Horizon Discovery, shares over 10 years of experience in gene modulation and gene editing technology and describes the unique features offered with the company's functional genomic screening services.
Screening innovation
Functional genomic screening looks at exploiting genomic editing to induce a measurable phenotypic change in a variety of organisms and tissues. This is broadly applicable to drug discovery workflows, from novel target discovery to target validation and better identification of compound mechanisms of action. While there are a number of screening platforms available, including the use of small interfering RNA (siRNA) and haploid genetic screening techniques, it is CRISPR technology that is currently revolutionizing biological sciences and opening up new applications of genomic screening.
CRISPR-Cas9 screening
Using the CRISPR-Cas9 system enables high-specificity, site-specific double-stranded DNA breaks to create out-of-frame edits or premature stop codons and effectively knockout a target gene. This technology can be incorporated into a range of screening approaches, from whole-genome studies to targeted drug resistance and sensitivity screens, to help answer complex biological questions and advance drug development programs.
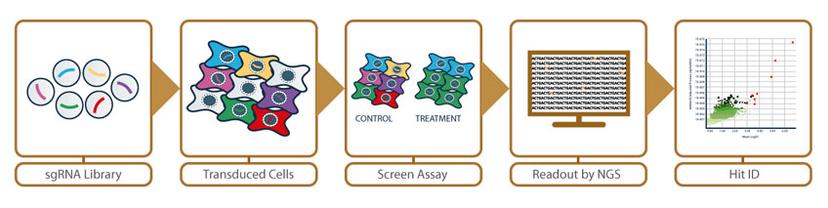
A typical screening workflow utilizes:
- A single guide RNA (sgRNA) pooled screening library – designed for a selection of target genes of interest within a pooled mixed population reagent
- Optimized cell lines – transduced with a pooled lentivirus library to screen against assay conditions
- Next-generation sequencing – deep sequencing readout to identify cell genotypes for hit discovery
From small-scale academic studies, all the way up to larger-scale complete workflow solutions, CRISPR screening enhances the discovery pipeline and provides valuable genetic insights.
Innovation in gene editing technology
Depending on the expression effect required, there are three types of CRISPR screen available from Horizon Discovery that can help to answer your research questions:
Type of CRISPR Screen Effect Application CRISPR KO Complete loss of target gene expression (knockout) This screening method provides a maximal window for inducing phenotypic change and provides high statistical power for hit discovery CRISPRi Repression of target gene expression Well suited to study druggability and to evaluate gene function with fewer sequence-specific off-target effects than other approaches (such as RNAi) CRISPRa Amplification of target gene expression Enables the study of activation-linked responses on a whole genome level and the overexpression of large transcripts not possible with some other approaches Dual CRISPRi/ CRISPRa Repression and overexpression of target genes Helps to identify components that influence resistance and sensitivity
What’s unique about the Horizon system?
JG: Horizon offers over 50 optimized cell lines and an impressive breadth of applications possible, and it’s still expanding. For a long time, screening was mostly limited to immortalized cell lines, but one of the great things that Horizon has been able to accomplish is to run screens in complex primary cells. This includes pooled screens in primary T cells and allows you to get data that is much more directly relevant for clinical applications.
Each type of CRISPR screening available from Horizon is a driving force by itself, but leveraging all three types (CRISPR KO, CRISPRi and CRISPRa) in dual screening really opens up the ability to look at both sides of the equation at the same time and see how compounds are really working and improve target discovery rates.
My career started at the bench, working for a small start-up, but I was inspired by field application scientists to help other people answer their own complex biological research questions.
James Goldmeyer Horizon Discovery
What are the research implications?
JG: There are almost limitless options in the screening workflow, meaning that researchers can address the questions that they really want to ask in specific models, such as primary cancer cells and even through in vivo screening. This breadth of capabilities means that we’re readily able to support both small and larger-scale studies with the same level of quality and commitment.
What do you think the future holds for this technology?
JG: Getting into primary T cells was a huge hurdle to overcome, but I think that there’s great potential to expand research into other hematopoietic cell types, neuronal cells and cells critical in disease development too.
We have a great set of tools for where we are now, but it would be great to advance the Cas9 platform to make the technology easier to work with, amenable to new delivery mechanisms and optimized for cell types not currently available. The great hypothetical question is: “Could we design a guide RNA that is able to both activate and inhibit, depending on what Cas9 form is present?” I hope that we can simplify the system to get more data from less resources, using fewer reagents to do the work.
Want to know more?
For more information on gene editing, screening and CRISPR technology:
- Visit our Gene Editing and CRISPR special feature
- Register for the webinar: CRISPR screening in primary human T cells
- Download the dual CRISPR screening poster
- Download Horizon’s screening selection guide
- Find out more about the effectiveness of CRISPR-Cas9 and synthetic crRNAs in high-content analysis screening
Does gene editing technology form a part of your work? We want to hear from you! Share your expertise.

