Manage every aspect of clinical trial lab data with a centralized database
Discover how a centralized lab database can improve decision-making in drug development by enabling real-time access to clinical trial data
24 Oct 2023

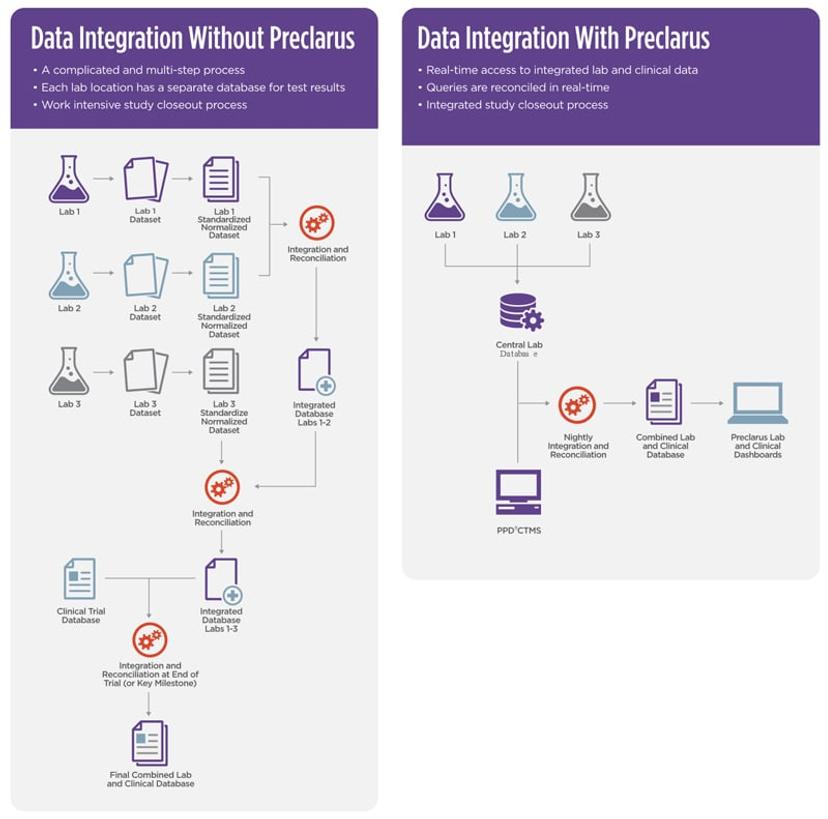
In drug development, clinical trials generate an avalanche of data that must be accurate and reliable. This data enables scientists to evaluate the safety, efficacy, and overall success of medical interventions so that they can reach informed evidence-based decisions. Traditionally, recording clinical trial data is a paper-based process, which raises several challenges. What’s more, each laboratory normally has its own separate database to record results, which makes integrating data from multiple sites a complicated and multi-step process, prone to errors and delays.
In this article, Chris Clendening, Senior Vice President, Global Laboratory Services, Central Lab, PPD clinical research business, Thermo Fisher Scientific, explains how the Preclarus® central lab database can help overcome some of the major challenges drug developers face during clinical trials by providing integrated lab data to project teams in real-time. Clendening covers what these challenges are and provides an overview of how Preclarus helps manage every aspect of the central lab’s role in clinical trials. Plus, he discusses what the future holds for this innovative database solution.
Challenges drug developers face with clinical trial data
Clinical trials present drug developers with a set of challenges that can be categorized into two groups: long-standing issues that have existed for decades, and new challenges that have emerged in the post-COVID era. Among the persisting challenges are issues like data currency. Clinical trials generate massive volumes of data at various stages, from patient recruitment to trial completion. Ensuring that this data remains current and accurate throughout the trial is an ongoing challenge, but this is crucial for making informed decisions, tracking progress, and meeting regulatory requirements.
Another major challenge is the chain of custody for samples. The movement of biological samples from trial sites to laboratories involves a complex chain of custody and traditionally, the lack of real-time visibility into sample movement results in delays and potential mishandling or loss of samples. The integrity and traceability of samples throughout this journey is essential for maintaining the reliability of trial results.
Clendening adds, “The economic landscape, especially in the wake of the COVID-19 pandemic, has introduced new challenges. Budget constraints and financial pressures have led to increased scrutiny of trial costs and the need for more cost-effective, speedy approaches."
Drug developers must find ways to streamline operations to ensure timely progress in clinical trials, without compromising trial quality.
Chris Clendening
PPD
Enter: Preclarus® lab solutions
The PPD laboratory services team has developed Preclarus®, an innovative solution to help scientists navigate the age-old challenges of drug development. This central lab database enables real-time access to laboratory data and results. It is designed to manage every aspect of central lab clinical trials, delivering reliable, integrated data to simplify and streamline workflows and enable faster more informed decisions.
The Preclarus platform serves a dual purpose. First, it provides tools for sites to manage various laboratory tasks efficiently and more effectively, such as sample access and logistics. It also shares real-time data through interactive dashboards and portals with clients, clinical trial sites, study teams, and clinical research associates (CRAs), enabling them to promptly oversee site activities, such as sample collection, query responses, kit orders, and potential areas of concern. This real-time overview enables more effective site logistics management, error resolution, and seamless operations to speed up the study closeout process. “The implementation of real-time query reconciliation has led to substantial time savings, with an impressive 400 hours saved per study. Additionally, this approach has resulted in a remarkable 67-80% reduction in queries per visit,” says Clendening.
The Preclarus platform significantly improves speed and efficiency in the drug development workflow by minimizing errors and error-related delays. By providing a single, web-based global database, it eliminates the need to merge and harmonize data, saving time and reducing the likelihood of errors compared to setting up multiple databases. It also enables real-time error checks during tasks like data entry and sample assessment which further helps prevent mistakes and speed up processes.
Clendening adds, “The system was purposefully designed to address common mistakes made by sites when entering information. By identifying the top 10 most common errors, the system assists users in making accurate decisions in real-time. As users log in samples, instant notifications flag inconsistencies with previous visits, resulting in up to 80% decrease in overall error rates compared to using paper-based methods.”
What do Preclarus users say?
Clendening explains that client feedback at the site level has been overwhelmingly positive, thanks to the inclusion of tools beneficial for sites and the attentive response to users' needs. He says, “A portal was developed through collaboration with investigators and clinical trial coordinators, to specifically address practical issues faced by sites. For instance, the introduction of a companion mobile app with biometric login removed the need for password memorization for multiple systems. The transition to paperless operations is also a huge advantage for sites as it simplifies all their activities and reduces site burden.”
Clendening adds, “Taking into account site feedback, we have expanded reporting capabilities to allow investigators to analyze data flexibly. We have also improved user experience by optimizing the application's layout, even incorporating features like immediate sample collection status updates.”
On the client side, the real-time monitoring capability Preclarus provides has been well-received. In today's complex clinical trials, tracking sample movements has become complicated, often spanning various labs beyond the central lab. Real-time tracking is crucial for managing these operations efficiently. The Preclarus platform addresses this challenge by providing visibility into sample collection, shipment, and delivery, ensuring a sample never gets lost.
Preclarus continues to expand its capabilities to help decentralize clinical trials
Clendening believes Preclarus holds broader potential beyond laboratory applications. The enthusiastic response indicates strong demand from both clients and sites, largely driven by the desire to shift away from paper-based processes. Looking ahead, the system aligns perfectly with the trend towards decentralized clinical trials.
“We are working on developing a patient-facing application to establish direct interaction with patients in home-based and near-patient settings while maintaining communication with medical professionals. The success of this hinges on providing patients with the right tools, coordinating clinical trial deliveries, and guiding them through the trial process. The next big step is determining an effective way to integrate Preclarus with all the other systems out there to offer a comprehensive, centralized solution,” Clendening says.
Clendening concludes, “With over a decade of product development under our belt, our established Preclarus system remains dynamic. Unlike static solutions, our product is under constant development by our dedicated IT team, and this process is driven by user feedback. Our commitment to ongoing upgrades stems from the belief that excellence is a journey, not a destination. This approach ensures that our product remains at the forefront of the industry, serving our clients with optimal efficiency.”

