Meet the microspheres helping to deliver rapid, low-cost, low-limit SARS-CoV-2 detection
Take lateral flow diagnostics to the next level with improved sensitivity for the future of IVDs
8 Jul 2021
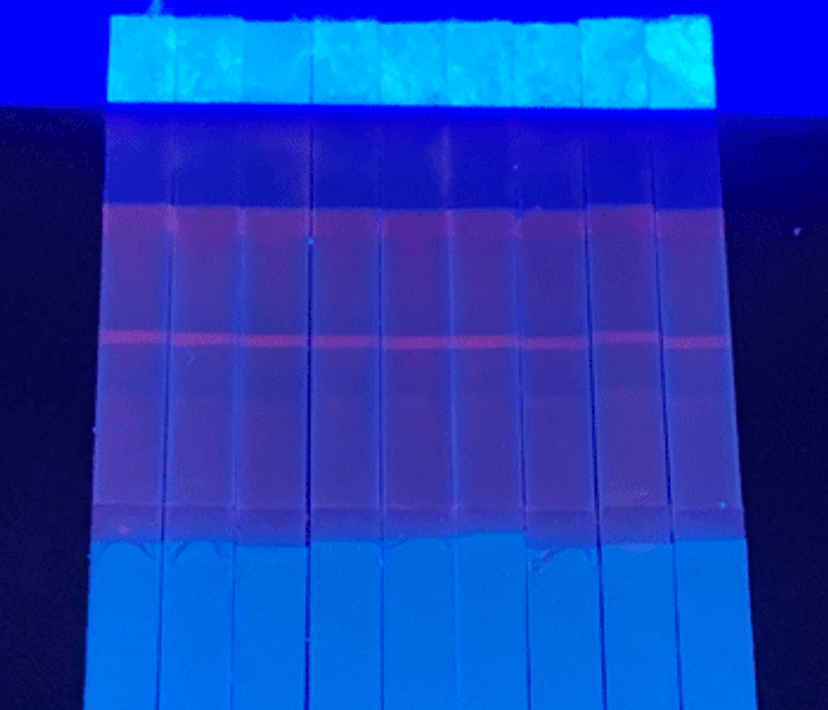
Explore the latest microsphere technology enhancing IVDs
At the beginning of the pandemic, there was a surge in demand for in vitro diagnostics (IVDs) as COVID-19 spread across the globe. From lateral flow assays to chemiluminescent immunoassays (CLIA) and immunoturbidimetric assays (IT), IVDs come in a variety of forms and can be used for many different applications, including the detection and/or quantification of hormones, antibiotics, autoimmune diseases, and microbial and viral infections such as SARS-CoV-2.
Despite their prevalence in the market, the development of lateral flow assays can be complex, with successful assay development requiring an understanding of multiple scientific disciplines. Most lateral flow assays are designed in one of four formats: sandwich, competitive, inhibition, or serum assays, each providing a different mechanism for the desired result. In this SelectScience® article, we explore the latest technology enhancing IVDs and delve into the real-life applications of this innovative technology.
Lateral flow testing
Lateral flow test strips have become an abundant IVD approach as they facilitate user-friendly, rapid, and low-cost analysis for clinical diagnostics. The latest developments in lateral flow tests have predominately focused on increasing the sensitivity of analyte detection and enhancing the sensitivity of quantitative tests using organic fluorophores.
Another game-changing innovation has been the improvement of advanced microspheres, small spherical particles that have a large surface area to help generate faster kinetics and lower detection limits. The surfaces of microspheres can be developed with different surface chemistries, providing better protein coverage and antibody orientation. However, there is a balance between sensitivity and assay run time that needs to be considered when selecting the right microspheres for your application. Larger microspheres increase assay sensitivity through increased detector particle-target analyte interaction time, yielding a higher sensitivity in exchange for a longer assay run time.
The importance of choosing the right microspheres
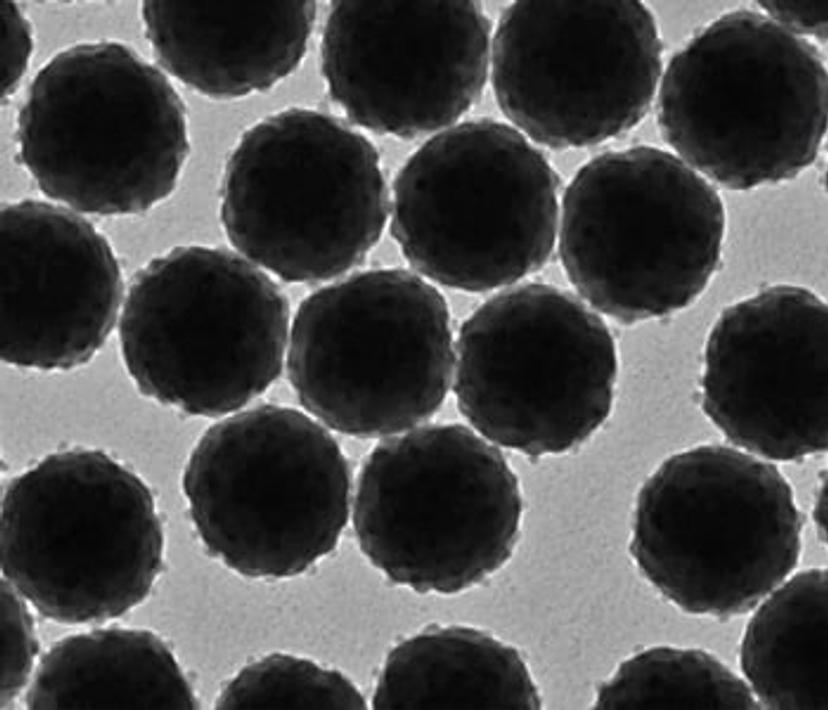
TEM analysis of Estapor® Europium Microspheres
Choosing the right microsphere is vitally important and the wrong decision could cost not only time but provide false results. To help mitigate this issue, further aid the streamlining of assay development and support the increased demand for COVID-19 lateral flow testing, the release of Merck’s Estapor® Europium Microspheres provides an optimized approach for lateral flow performance.
Labeled with europium, these microspheres provide several advantages over traditional fluorescent microspheres. Estapor® Europium Microsphere’s exhibit an enhanced fluorescent quantum yield, facilitating a reduced limit of detection to significantly improve the sensitivity of lateral flow testing, which in turn assists in removing false-negative results and prevents further spread of disease.
AnteoTech case study: A new alternative for bio-conjugation
One company utilizing this technology is AnteoTech, a Brisbane-based, ISO 9001 and ISO 13485 accredited company that produces AnteoBind™. Unlike common bio-conjugation options such as passive absorption and conventional covalent chemistry, AnteoBind™ uses metal ion polymers to help bind antibodies and proteins to stable, activated surfaces via active binding. This enables effective and reproducible results that can be scaled up – a vital advantage for mass production of clinical diagnostics.
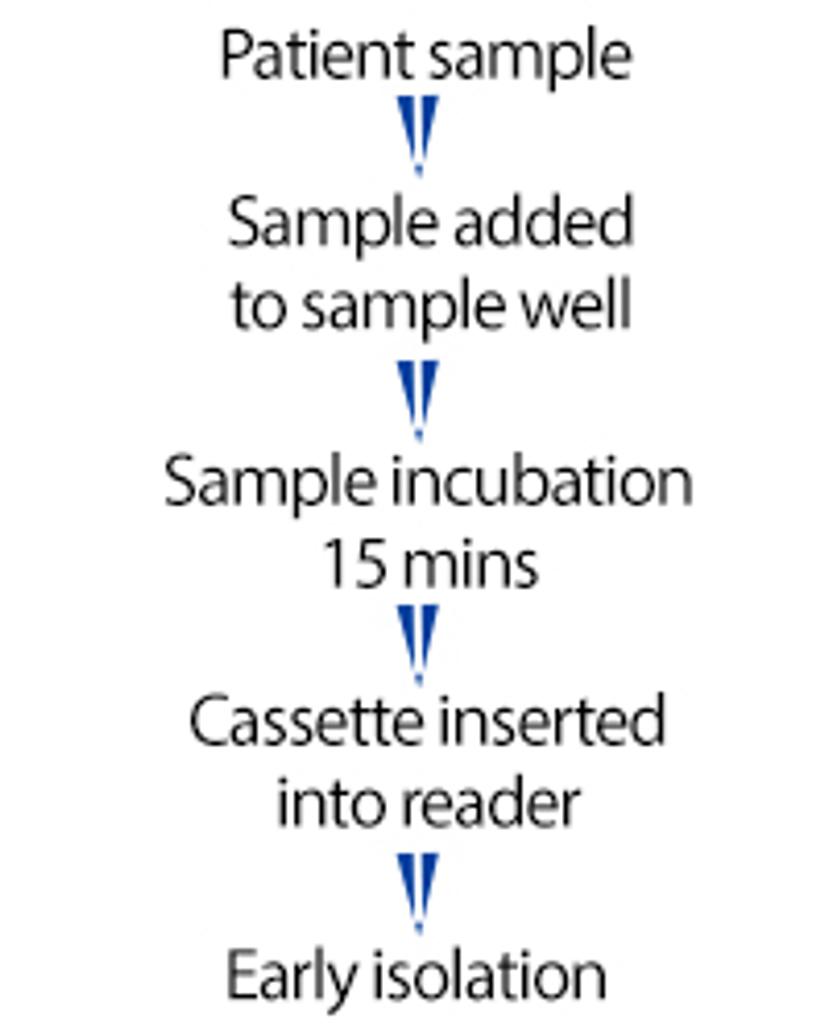
AnteoTech's 15-minute SARS-CoV-2 antigen RDT workflow
AnteoBind™-Estapor® Europium Microsphere conjugation is a four-hour process with simple activation, conjugation, blocking, and storage steps. This short process offers many benefits as particles have the same size distribution but different surface charges through the bio-conjugation process. Activated AnteoBind™ particles have a positive zeta potential compared to stock Estapor® and cTni-conjugated particles with negative zeta potential when looking at cardiac troponin I antibody conjugation. This data provides evidence of successful activation and conjugation.
AnteoTech has been able to successfully replicate a SARS-CoV-2 antigen rapid diagnostics test (RDT) workflow with activated Estapor® Europium Microspheres with extremely low limits of detection of viral antigen at 20 pg/mL. From on-site sample collection and lysis buffer mixing to cassette processing and test results, this process takes only 15 minutes, which enables early isolation of infected patients and a reduced community transmission rate.
This technology has the potential to test for multiple proteins and antibodies in one strip, enabling the testing of multiple diseases at once. AnteoTech has produced a proof-of-concept lateral flow assay with Estapor® Europium Microspheres to detect multiple viral antigens in relation to FluA and FluB as well as biomarkers (e.g., interleukin-6 and procalcitonin) produced in reaction to sepsis. This assay provided good signals for the multiplexed assay in buffer or serum and no cross-reactivity was observed between antibody pairs for their respective antigens. These studies provide further evidence for working assays with the ability to screen multiple diseases, with lower detection limits up to ten times lower than common protein particle bio-conjugation options such as colloid gold-based assays.
The future of point of care
The combination of Estapor® Europium Microspheres and AnteoBind™ has the potential to revolutionize point-of-care testing as this pioneering technology is designed to:
- Reduce antibody screening and optimization time during assay development from months to just weeks
- Demonstrate easy blocking with no cross-reactivity in multiplexed lateral flow assays
- Retain antibody activity with high sensitivity
- Be used with conjugate sizes of PDI <0.1 to allow for easy reproducibility
All of these factors facilitate scaling up processes which are essential for mass distribution.
For more information on the design, application, and manufacturing of lateral flow tests, see the related resources below.
Application note: Estapor® microspheres for lateral flow
Articles:
The Life Science business of Merck KGaA, Darmstadt, Germany, operates as MilliporeSigma in the U.S. and Canada

