Navigating the complexities of cell-based assays in GMP QC testing: The journey to robust assays
Deepen your understanding of cell-based assays and the process of developing robust cell-based assays for GMP QC testing in modern drug development
13 Aug 2023To ensure pharmaceutical product quality and efficacy in therapeutic development, it is important to use mechanism of action reflective biological activity assays that can be validated against good manufacturing practices (GMP). Cell-based assays play a crucial role in modern therapeutic development. Utilizing reliable cell-based assays in your GMP quality control (QC) testing ensures a consistent and comprehensive assessment of drug efficacy, providing confidence in the safety and quality of end pharmaceutical products and are strongly recommended by regulatory authorities. The design, development, and validation of the cells going into your assays is, therefore, instrumental when working in a regulated environment and must not be overlooked.

Cell-based assays are bioassays that take place within living cells and can be categorized into cell proliferation, cell death, and cell signaling assays.
These assays offer several advantages:
- The ability to mimic the drug's intended physiological environment, providing biological relevance
- Comprehensive assessment of drug function beyond binding interactions, enabling a better reflection of complex mechanisms of action
- Offering customizable assay designs that align with regulatory preferences
Cell-based assays are key in modern drug development and bridge the gap between in vitro and clinical outcomes, capturing specific interactions and functional responses accurately. Regulatory agencies recognize the value of cell-based assays for potency determination and encourage their appropriate use.
Developing robust cell lines for use in establishing cell-based assays for GMP QC testing involves various steps to ensure quality and reliable performance. The initial process of developing robust cell lines can be divided into distinct phases depending on the objectives; These phases include planning and design, development, cell line characterization and validation and subsequent GMP assay validation.
This article will explore how a cell line is designed, developed, and validated for research use and provide an overview of the requirements for GMP QC testing, with insights from experts at Svar Life Science, a company that promises robust and reliable assay technology and applies the best analytical technologies for drug development and clinical diagnostics. Optimizing GMP QC testing with physiologically relevant cell-based assays can accelerate therapeutic development, ultimately contributing to a more promising future in healthcare.
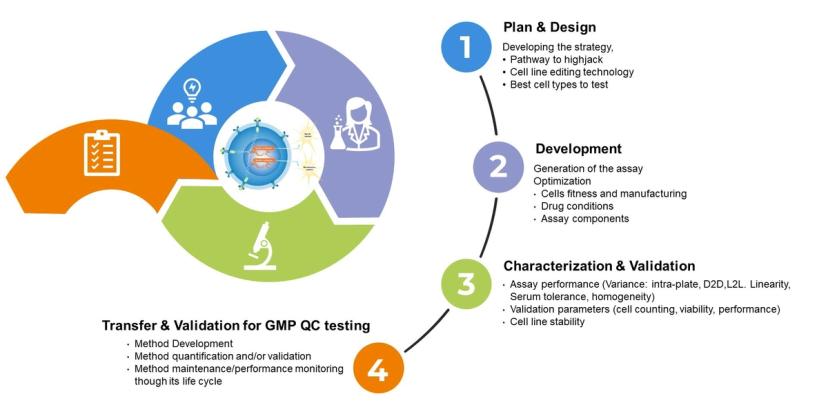
The stages to developing reliable cell-based assays for GMP QC testing
1. Planning and design: Mitigate potential challenges
The planning and design phase is crucial for developing and validating cell-based assays. Having a detailed understanding of the desired end product is key to overcoming and avoiding potential challenges during the development and validation of these cell-based assays. Thus, ensuring confidence that your cell-based assays provide accurate results for GMP QC testing, optimizing the drug development process and accelerating therapeutic advancements.
“It is important to consider the intended use and type of assay, such as high-throughput screening, functional assays, or toxicity assays,” explains Dr. Jordi Rodó Morera, Global Innovation & Scientific Lead, at Svar Life Science. “We also need to clearly identify and define the development pathway to fully understand the molecular basis that we are working with.” As cell-based assays are designed to closely mimic the mode of action exhibited by the drug being tested, this step is important because it allows us to align the assay with the specific molecular targets and mechanisms of action related to the drug being tested.
Rodó Morera continues, “at this stage, we also need to consider the molecular tools at our disposal to manipulate the assay, for example, to express luciferase. Additionally, the desired sensitivity of the assay for testing the specific drug should also be considered.” Once this information has been gathered, an appropriate approach for editing the cell line can be selected. Various technologies are used for genetic manipulation. Molecular tools such as CRISPR and TALEN allow precise genome editing, while lentiviral vectors and transposons help integrate genes into cells. However, gene integration tools may not be as specific as genome editing tools. Another technique, transient transfection, can be used to create short-term cell-based assays, but it is mainly limited to research and development purposes and is not commonly used for GMP QC testing. Finally, the cellular background of the assay is a crucial consideration in the design and planning stage, which is determined by the drug's intended use. The type of cells chosen should be influenced by the type of drug and the mechanism of action. Various cell lines are available from Svar Life Science, and the selection depends on the specific assay requirements.
2. Development: Identify, edit, and optimize
“Once the design strategy is defined, we can transition to the next phase, which is the development of the cell-based assay. During development, high-throughput screening methods are used to modify and identify the best cell lines to develop the assay. A meticulous development process is vital for generating fit-for-purpose, GMP-validated cell-based assays, which play a crucial role in the potency testing of your therapeutics.
Rodó Morera explains, “assay development involves employing the appropriate tool to modify the genome of our cell line chosen during planning and design.” Cell lines can be tested in parallel based on the cellular background. For instance, if a B cell background is desired for the cell-based assay, both Raji and Ramos cell lines can be modified accordingly.
Svar Life Science develops custom assays as well as assay ready services for a wide range of targets and customers
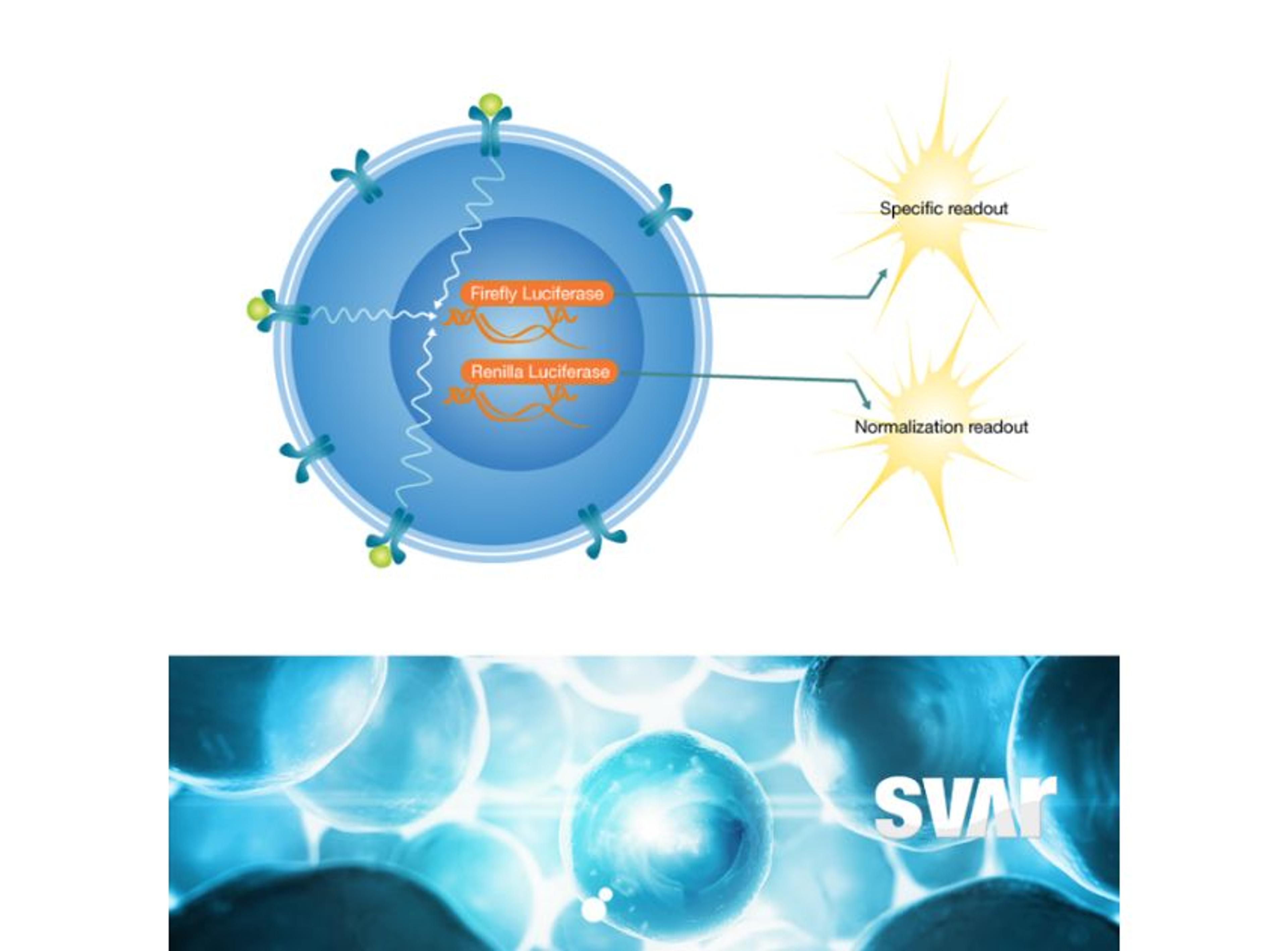
Following genetic modification, the chosen cells will be isolated and expanded. The different clones will then undergo genotypic and phenotypic characterization to assess their genetic and observable traits. Rodó Morera explains, “we can select, and we can identify the clones with higher dynamic range, which are the ones that we select to use as a cell-based assay further on”. Dynamic range describes the range of concentrations or values within which an analyte can be accurately detected and quantified.
“Once we have chosen the cell line, it's time to begin the optimization process,” says Rodó Morera. “This optimization will occur on several fronts, namely cells, drug conditions, and assay components.” For instance, bioassay fitness can be enhanced by fine-tuning various cell-related factors, such as cell confluence in the plate. Using assay-ready cells can improve efficiency, minimize signal variance, and ensure reproducibility. These cells are prepared individually in vials and subsequently thawed and plated with the drug. The choice of freezing media for assay-ready cells is crucial for maintaining functionality and viability.
Next, it is important to address critical parameters affecting the cell-based assay from a manufacturing perspective, including culture conditions, incubation periods, and optimizing drug conditions. This optimization involves determining the appropriate concentrations, response curve, incubation durations, and evaluating the use of serum components, especially for drug development targeting the complement system. “Finally, the assay controls, biological matrix, detection reagents, and equipment will all be optimized,” Rodó Morera explains. “This comprehensive approach allows us to develop a cell-based assay successfully.”
3. Cell line characterization and validation: Ensure proper functionality
Following the development of the cell-based assay, the cell line must be characterized and then validated to ensure proper functionality of the assay. Ensuring the assay works as expected is crucial to optimize drug development processes and expedite therapeutic advancements effectively.
Rodó Morera explains that at Svar Life Science, characterization consists of a series of experiments designed to determine factors including the variance and accuracy of the cell-based assay within the plate, from day to day, and from lot to lot. “We also test and characterize the linearity, which is especially relevant for potency assays,” he continues. “Moreover, the behavior of the cell-based assay in presence of serum is normally also tested, as well as the homogeneity of the plate to avoid any undesired edge effects.”
Throughout this characterization process, several parameters will be established as cutoff values and subsequently employed for validation purposes. Validation includes multiple experiments over several days, and with different operators to help identify any potential sources of variability. If all these parameters are met, the cell line will be validated.
Some specifications derived from this characterization and validation process are regularly utilized to assess the stability of each produced cell line lot. If any of these parameters fail, the respective lot is removed.
“It is worth noting that this process is not linear but rather circular,” comments Rodó Morera. “The knowledge gained during the development of one assay plays a crucial role in the development of subsequent assays. Once all three phases are completed, we have a validated cell-based assay for research use only,” adds Rodó Morera. “The next step involves transferring this assay to the GMP QC team for further validation for GMP application.”
4. Transfer and validation of assay for GMP QC testing: Partner with an expert
Cell-based assays play a valuable role in various stages of modern drug development, including QC potency testing. When a cell line has been well characterized and validated, the cells can be used within the QC testing services in developing a potency cell-based assay, which will then be used for release testing on pharmaceutical products. QC potency testing is an essential aspect of the QC procedures performed under GMP.
“A potency assay for GMP QC testing needs to be able to detect changes in biological activity of the drug compound, meaning the assay should be stability-indicating, comments Dr. Azra Alajbegovic, Scientist at Svar Life Science, with much experience in cell-based assays for GMP quality principles. In order to use the assay for GMP QC testing, it is required that the assay goes through a thorough validation. This includes method development, method qualification, method validation, and ongoing performance monitoring. Each of these stages presents unique compliance requirements. “Using robust cell-based assays can give you confidence that your results are accurate and comply with regulatory standards for GMP.”
By partnering with an expert in cell-based assays, you benefit from specialized expertise, advanced technologies, and regulatory compliance.
Dr. Camilla Melin Fürst Svar Life Science
GMP QC testing is a comprehensive process requiring careful consideration of multiple factors to ensure the development of safe and efficacious therapeutics. “It's crucial to document all aspects of assay development, validation, and performance in order to ensure compliance with GMP regulation,” adds Dr. Camilla Melin Fürst, Senior Scientist and Project Manager, Svar Life Science. “In order to make this a smooth process, it can be useful to have an experienced collaboration partner."
Partnering with specialist companies has become a common practice in this challenging regulatory landscape as it allows companies to access specialized regulatory and scientific knowledge. Melin Fürst adds, “by partnering with an expert in cell-based assays, you benefit from specialized expertise, advanced technologies, and regulatory compliance”.
The design, development, and validation of your cell-based assays are crucial considerations for both QMP QC testing and overall drug development processes. These assays play a significant role in modern drug development, providing valuable biological information that reflects the mechanism of action. Ensuring the quality and efficacy of your pharmaceutical end product heavily relies on this data. Having confidence in the robustness and reliability of your cell-based assays instills confidence in your GMP QC testing, paving the way for therapeutic advancements and the betterment of human health.
Learn more about how the iLite® Custom Development & Cell-based Services from Svar Life Science can aid your GMG QC testing processes>>
Want more? Learn more about the use of cell-based assays in infectious disease research, biosimilar development, and bispecific antibody development