Organoid library development: Testing patient sensitivity to potential cancer therapies
Discover how one lab is using patient-derived pancreatic organoids to deliver personalized treatments
1 Oct 2019
The ability to cultivate miniature cellular systems in vitro has become a powerful tool in disease modeling and drug discovery. Organoids are cultured from stem cells within a 3D medium to provide a more physiologically relevant and realistic view of cellular interactions than can be seen with traditional 2D models. These 3D models are used to study how cells interact with each other within an organ, to gain insight into how disease processes disrupt these interactions, as well as to test the impacts of potential treatments.
In this article, Dr. Hervé Tiriac, associate project scientist at the University of California (UC), San Diego, discusses his role in creating a pancreatic organoid lab for testing cancer therapeutics in vitro.
A personalized approach to cancer treatment
If you can grow a patient-driven model, such as an organoid, you should be able to use it as a tool to help guide therapy for that patient.
Dr. Hervé Tiriac has dedicated his career to fine-tuning the protocols for establishing pancreatic organoids. Building on his research at Cold Spring Harbor Laboratory, Tiriac is currently working on translating these protocols for clinical use.
Organoids are promising tools for use in precision medicine, particularly for the development of individualized treatments. Through use of a library of close to 70 patient-derived organoids, Tiriac has demonstrated that organoids reflect the molecular details of the tumor from which they were derived. Through his extensive research, Tiriac also reports that, if a patient's organoids are sensitive to therapies in vitro, then the patient tends to be sensitive to the same drug given in the clinic. This robust predictive power can be a useful tool in identifying the best treatments for patients on a case-by-case basis.
The Department of Surgery at UC is now working on kickstarting Tiriac’s research by growing its own pancreatic organoid library to test patient reactions to potential cancer therapies.
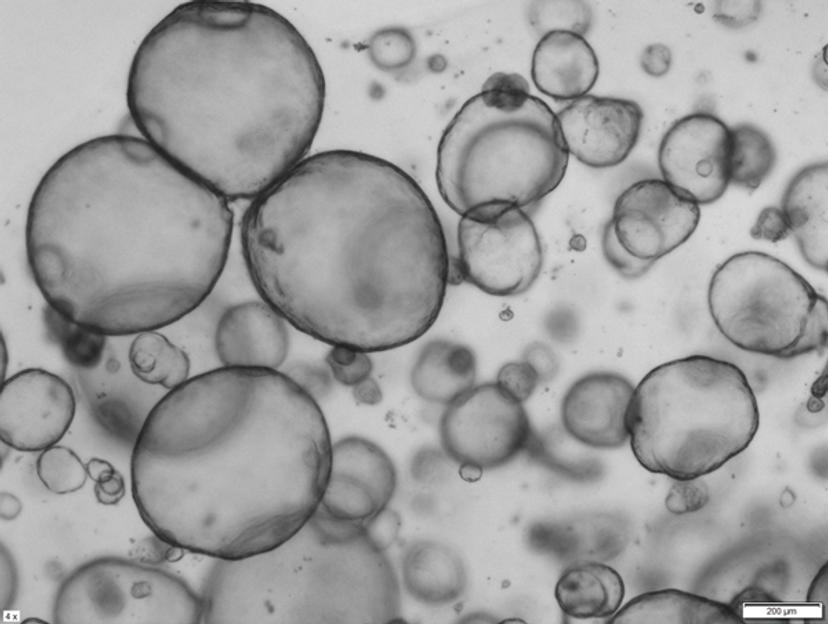
From tumor biopsy to 3D modeling
Top tips for human organoid development from the expert:
1. Familiarize yourself with existing protocols and choose your media to match your cell type
2. Take the recommended quality control steps to ensure your media can stimulate sufficient growth
3. Start off practicing with mouse models to develop your technical skills
The foundation for UC's organoid library is based on surgical and endoscopic ultrasound fine-needle biopsy (EUS-FNB) samples from patients with pancreatic ductal adenocarcinoma (PDA). Once a tissue sample is received, it is enzymatically digested, directly embedded into a 3D environment, and fed with growth factor and Wnt ligand-rich media that stimulates cancer cell proliferation and organoid formation.
This media often has to be generated on site, so it can take a few months to prepare everything. Tiriac clarifies: "It's better to use homemade conditioned media that is taken directly from the cells that produce the required Wnt3A ligands. So, it does take time." The resultant medium, referred to as human complete feeding medium, contains a mixture of components, including the Wnt3A ligands, Noggin, nicotinamide, and R-spondin 1.
Tiriac's lab then plates multiple domes, a 3D mix of cancer cells and Matrigel, into 6-well dishes containing this media. Tiriac notes: “We also use Corning HYPERFlasks, which provide ten times the growth surface of a T175, in a single dish. Basically, using one culture dish instead of 10 has allowed us to produce larger batches, faster, whilst using less tissue-culture real estate."
The remaining challenges for pancreatic organoids
Despite being a powerful tool, organoids present their own challenges. The main limitation is that they are unable to exhibit all the features and interactions that would occur in a living, breathing organism. When asked how this impacts his work, Tiriac explained: "We know that, for many cancers, the environment is what stimulates the growth of cells. However, we're not really modeling that in the dish. So, one challenge is creating a protocol where there are more cell types working together and influencing each other, just like they do in the body."
Tiriac also highlights that, to build a useable organoid library, the department requires sufficient patient samples that contain living cancer cells, but a lot of patients receive neoadjuvant treatment, meaning treatment before the tissue is taken by surgery. "If this works, it's fantastic for the patient, but it prevents us from being able to model the disease in the dish. Unfortunately, with chemotherapy at this stage, the disease often comes back. So, we know that not all the cancer cells are killed, but our technique is not currently able to capture those few remaining cells and grow organoids from them."
I hope to continue to use organoids to identify and differentiate drug-sensitive and drug-resistant patients and determine biomarkers for these traits.
Ambitions for future organoid libraries
The restart at UC has been successful in their focus on pancreatic organoids. However, there is the ambition to expand this concept to test treatments for a wider range of diseases. The next step is to take the approach to other gastrointestinal cancers, for example developing colorectal organoids, where the protocols are robust and the approach is similar to what is currently being employed.
Tiriac went on to express his desire to optimize the process further, explaining the need for more rapid identification of the best treatments: "Something that could really benefit one patient, might not benefit most patients with the disease. So, currently, making an organoid for every patient is something we have to do. However, in the future, we should be able to determine what makes a patient sensitive or resistant and establish a rapid test, without requiring an organoid for every single patient."
Find out more of the latest advances with our 3D Cell Culture special feature>>
References:


