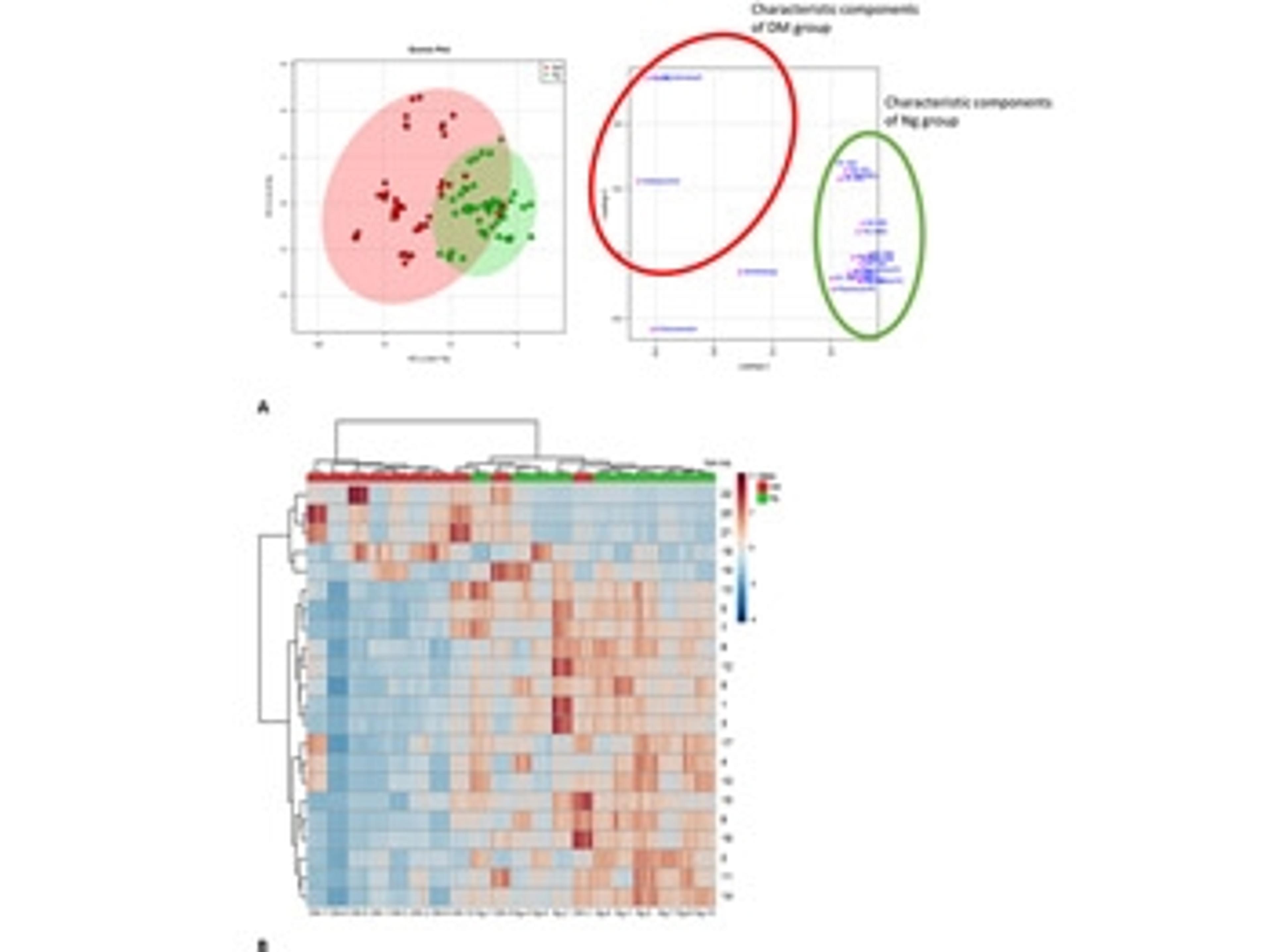
bioMérieux receives US FDA 510(k) clearance for its AST System VITEK REVEAL
bioMérieux, announces that its VITEK REVEAL AST System, reporting results directly from positive blood cultures, has received US Food and Drug Administration (FDA) 510(k) clearance