Cell Line Development Feature
Cell-based assays are crucial for biologics development but face challenges like variability and complex setups. Learn practical tips to reduce assay variability, improve reproducibility, and generate regulatory-ready data for next-gen therapies.
Download resource
Teva Pharmaceuticals achieved higher throughput and faster data collection with the BioPhase 8800 system, maintaining data quality and existing SOPs. Explore how this innovation supports stability testing and process development.
Download case study
Count a wide range of cell types without slides using the CellDrop™ FL, an automated cell counter for cell culture, primary cells, stem cells, organoids, hepatocytes, yeast, and more. Experience innovation in action.
Watch video
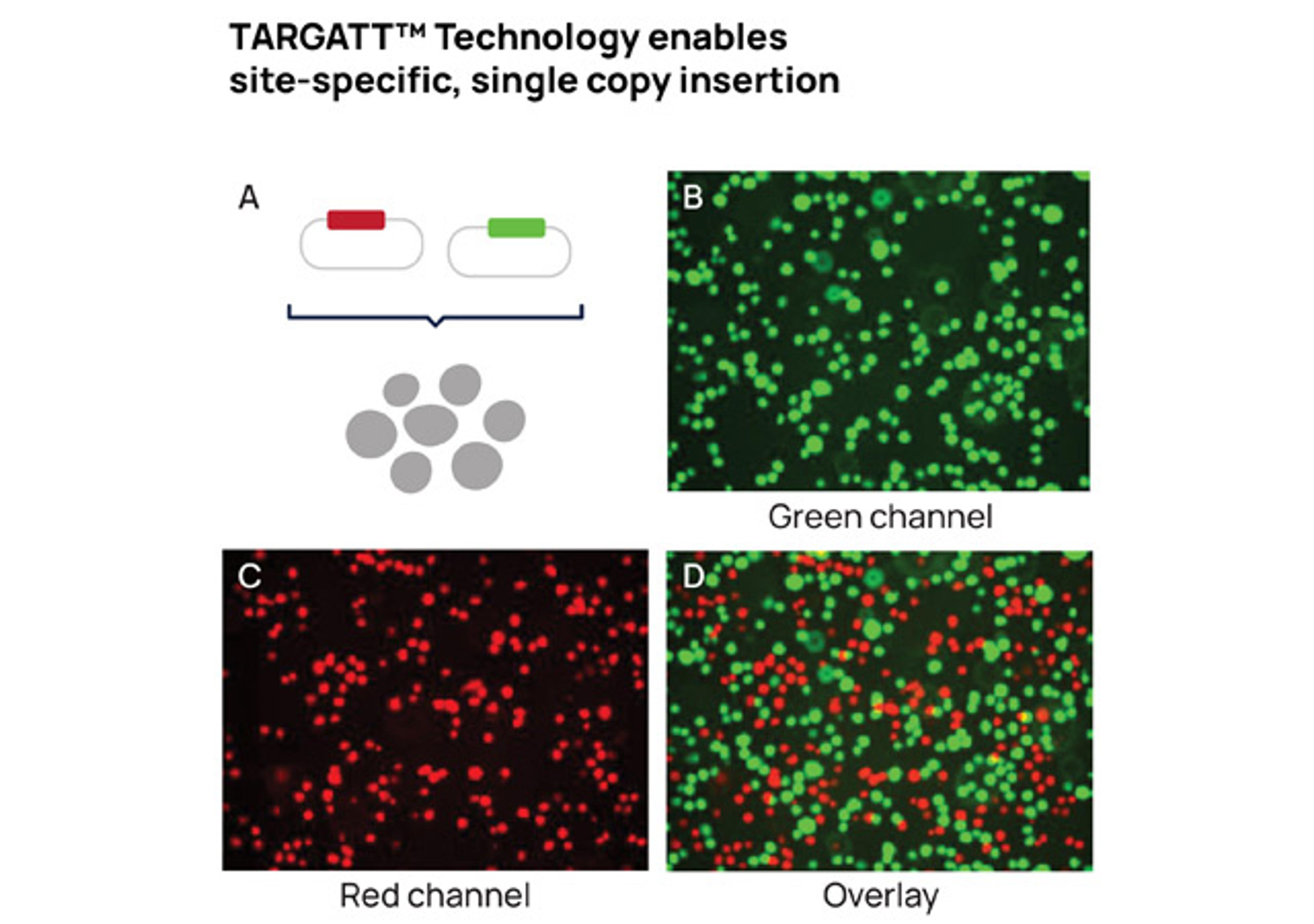
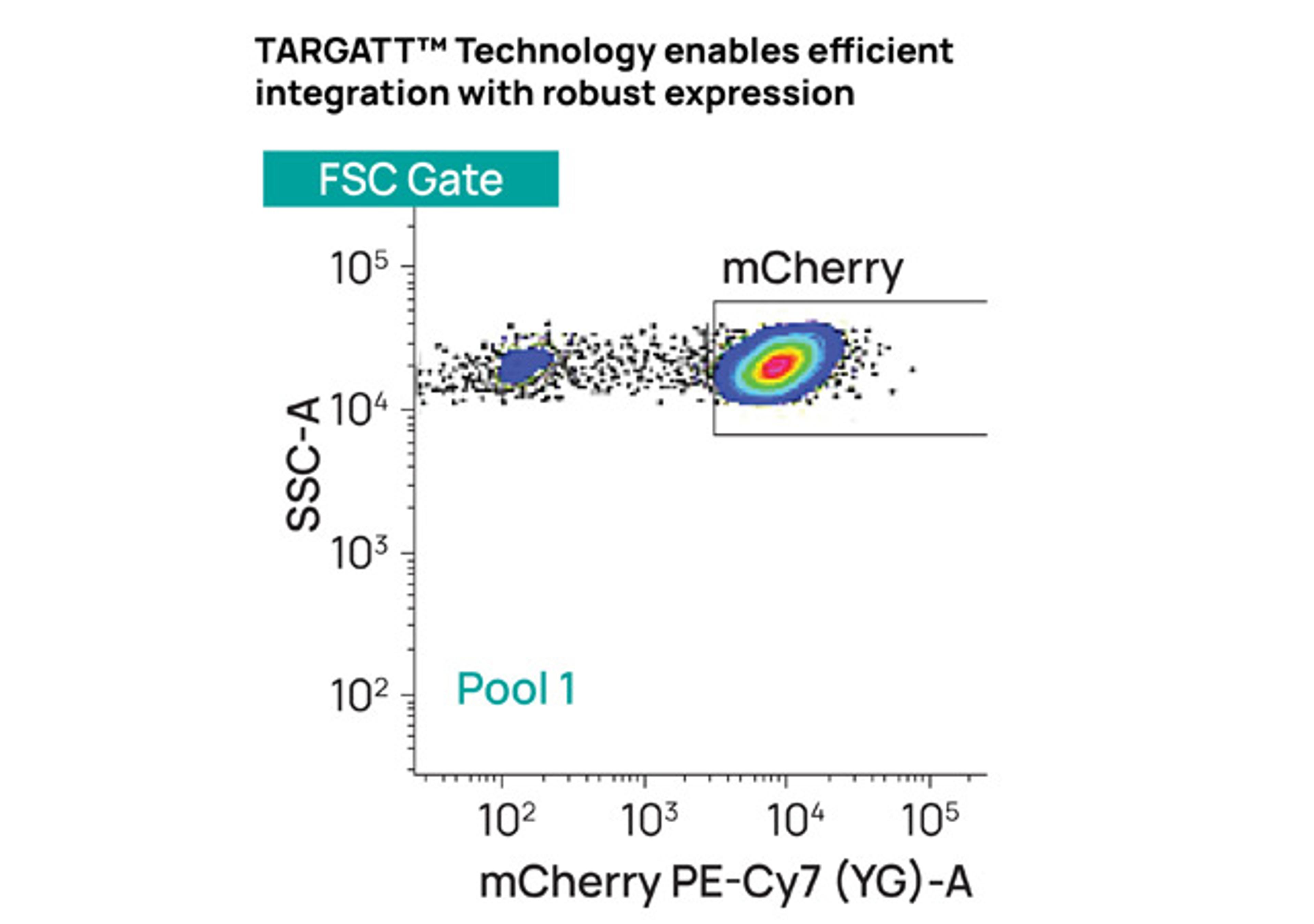
Accelerate cell line development with TARGATT™ large knock-in technology. Enabling site-specific single-copy insertion of 20+ kb of DNA with high efficiency and negligible off-target events.
Download resource