Enhancing the Lives of Children with Tandem Mass Spectrometry
How scientists at Birmingham Women’s and Children’s Hospital have integrated the latest advancements in clinical mass spectrometry to provide better treatment for serious metabolic disorders
11 Jul 2018
For almost 160 years, the Birmingham Women’s and Children’s Hospital, UK, has provided a pioneering health care service to its patients across the West Midlands. With a rich and impressive history of world-leading innovations, honored in a recent documentary celebrating 70 years of the National Health Service, the trust has a firm vision to provide unparalleled hospital and community care.

Within the newborn screening and biochemical genetics lab, clinical scientists are using top technologies to identify inherited metabolic disorders that could otherwise prove fatal. We spoke to Dr. Philippa Goddard, consultant clinical biochemist, to find out how LC-MS/MS, in concert with the latest software advancements, have helped to achieve streamlined clinical workflows that have significantly benefitted patients with the conditions included within the UK newborn blood spot screening program and in some cases have saved lives.
The technology has really come to fruition over the past decade
Dr. Pippa Goddard Consultant Clinical Biochemist, Birmingham WCH
SS: Please introduce yourself and your place of work
Pippa Goddard: I’m a consultant clinical biochemist at Birmingham Women’s and Children’s NHS Foundation Trust. I’ve been working in clinical biochemistry for the past 20 years, and 15 of those have been spent here at the West Midlands Newborn Screening and Biochemical Genetics lab. Tandem mass spectrometry is indispensable to our high-throughput screening program, which covers the analysis of approximately 75,000 dried blood spot samples a year. Whilst working here, I’ve seen the introduction of mass spectrometry for an expanding compendium of metabolic disorders, so the technology has really come to fruition over the past decade, enabling us to detect rare conditions that can be treated and managed in a timely fashion. Through our multidisciplinary work, we hope to reduce morbidity and mortality of all patients identified through the screening program.
SS: Why is tandem mass spectrometry, LC-MS/MS so important to the work being conducted in your laboratory?
PG: Since we’re the primary screening facility for the West Midlands region, we analyze about 300-400 blood spot samples a day. With this in mind, we need a system that facilitates rapid measurement of many metabolites in a small amount of sample; the concentrations of which are used to identify various metabolic disorders. As advances in sample preparation and data management have emerged, the capabilities of modern mass spectrometry technology have truly met the demands of our clinical laboratory. Previously, we would derivatize samples and manually transfer them into separate microtiter plates. Now, we can simply place underivatized samples directly onto the mass spectrometry machines. We run the samples on two identical Waters ACQUITY™ UPLC™ I-Class / Xevo™ TQD IVDs overnight, so the results are all ready to evaluate first thing the following morning. In essence, we wouldn’t be able to analyze the quantity of samples that meets the quality of national guidelines, without these tandem mass spectrometers.
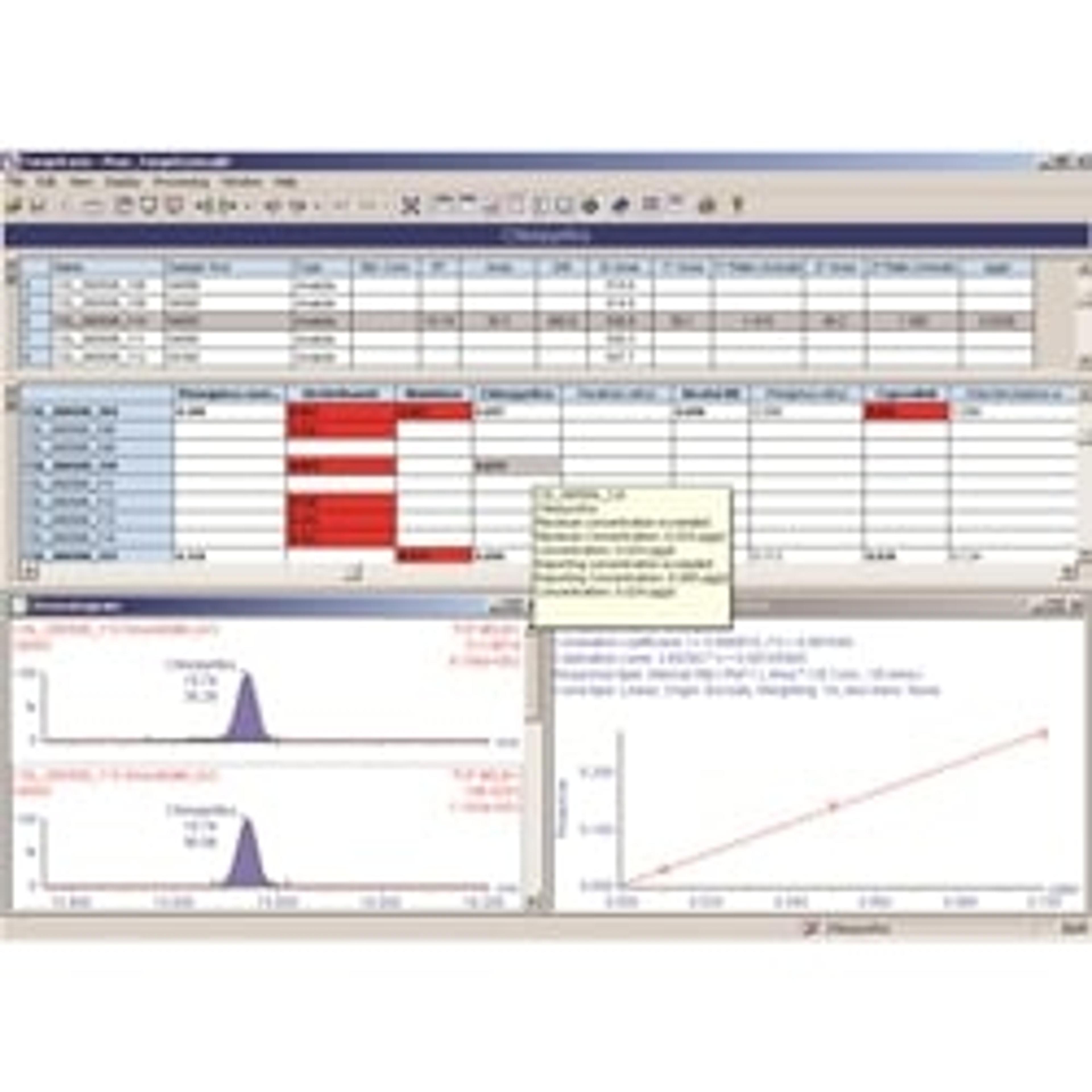
SS: Talk me through how data management is embedded into your workflow
PG: We use a combination of different data management platforms in a bi-directional fashion in order to acquire, correlate and analyze our clinical data accurately. We use a screening information management system (SIMS) called OMNIclient, in conjunction with demographic download from our regional child health record department, as well as the MassLynx™ Software from Waters.
First, we collate all the sample information; each sample carries barcoded episode numbers for easy identification. These lists are then imported into our SIMS, which creates worksheets recognized by MassLynx for sample analysis on the mass spectrometry platform. When the sample run is complete, there is an automated upload of results onto the server, where they are married with the patient demographics.
We wouldn’t be able to analyze the quantity of samples that meets the quality of national guidelines without these tandem mass spectrometers
Dr. Pippa Goddard Consultant Clinical Biochemist, Birmingham WCH
There is a series of background rules, which we have defined according to national standard cut-offs, in the SIMS that enables auto-verification of samples. In fact, there is a two-stage validation and authorization process which means that numerical values from the machine are cleverly matched to our standard qualitative codes. Any results above our user specified reference limits, i.e. in the ‘condition suspected’ category, are checked, acted upon and, if necessary, the appropriate treatment regimen implemented for that patient. MassLynx enables us to check the detailed flow profile of all our samples, which are important to cross reference against the numerical values. Together with evaluating the peak intensities and internal QCs of the samples, this gives us a good indication of whether they injected properly. We also use the numerical data from MassLynx to compare samples against patient population means, as a further verification step.
SS: Are there any challenges you’re looking to overcome, or future developments you’d like to see, in your mass spectrometry workflows?
PG: In the UK, we’re largely guided by the national screening committee and strict UKAS regulations, so our method development is limited, but we’re frequently involved in pilot studies for expanding the use of mass spectrometry as a diagnostic tool. The robustness of Waters’ platforms, and their user-friendly interfaces, make the process of introducing new metabolites relatively simple, once the standards have been verified and validated.
Find out more about Waters' family of LC-MS/MS in vitro diagnostic medical devices