Your Ultimate Antibody Guide: Best Practices and Top Tips
Learn how to choose the best types of antibody for your application, plus discover top tips for antigen and antibody optimization and validation
20 Nov 2018
Antibodies are unique glycoprotein complexes that are a product of adaptive humoral immunity, produced in response to foreign pathogens and particulates. Over the last few decades, they have become an invaluable tool in basic and translational scientific research, enabling physiological processes to be explored and characterized at the molecular scale, in time and space. This article will guide you through a comprehensive compendium of top tips and best practices in antibody selection, validation, and assay optimization, with downloadable PDF put together by experts from Biorbyt.
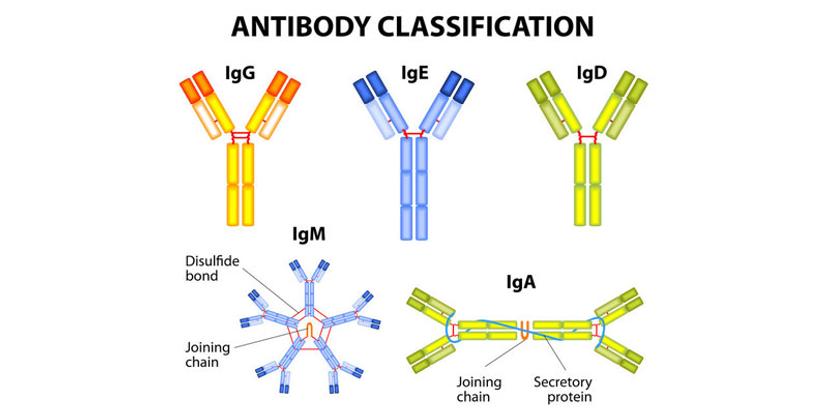
Comprised of four polypeptide components — two heavy and two light chains arranged into an interesting Y-shaped symmetry — antibodies bind to a complementary epitope sequence via the variable region, located on the terminal ends of the heavy and light chains. In nature, antibodies are produced with an affinity to a variety of epitope sequences on one antigen, with varied specificity. Yet, the ability to synthetically manipulate antigen immunogenicity and improve binding specificity during the production process makes them a particularly useful tool in research.
Bound to fluorescent or chemiluminescent probes, antibodies can be used to identify protein, peptide, or DNA/RNA sequences in a variety of sample types. Today, monoclonal antibodies (mABs) are also being developed to treat diseases, such as cancer and autoimmune disorders. Monovalent antibodies can be raised to target surface antigens specific to a target cell population, blocking key signaling axes to mediate cell behavior. They can be designed to become internalized and introduce a conjugate, often cytotoxic, drug molecule into a target cell.
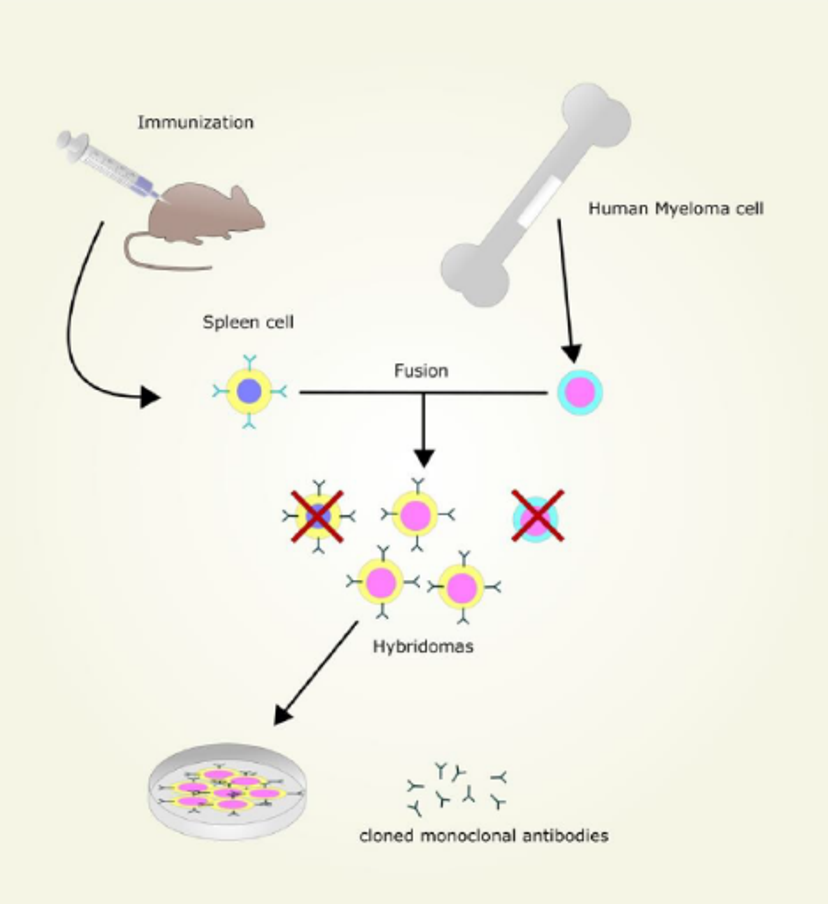
A brief history
In 1975, Milsten and Kohler founded the hybridoma technique, and introduced monoclonal antibodies to the life sciences field. By harvesting B-lymphocytes from mice spleens and fusing them with immortal myeloma cells, they found that they were able to generate clonal antibody-producing B cells. These antibodies could then be separated into clones and screened for immunogenicity.
This method of production was so robust that it has remained a primary method ever since. The phage-display method, introduced in 1986, enabled screening of recombinant engineered isotopes for specificity when fused to a carrier phage protein. As new methods continued to emerge, customization of the constant region and immunoglobin fragmentation could also be achieved – improving speed, reducing cost and fine-tuning specificity further.
Download this PDF to learn more about the hybridoma technique, and the history of antibodies >
What makes a great antigen?
There are a multitude of antigen types that can be used for antibody production. Selecting an antigen that elicits a highly immunogenic response, that is also pure and non-toxic, is vital. Whole proteins are optimal antigens since they have a high molecular weight, and are often conjugated to a cleavable HA, His, or Strep tag for screening purposes. Peptides, usually of 10-20 amino acids in length and conjugated to a carrier protein, are also often used to produce side-chain specific antibodies that detect post-translationally modified proteins in cells or tissues. The detection of phosphorylated protein derivatives, for example, has become particularly important in molecular research. Generally, the longer the peptide, the greater the immune response, but this comes at a cost of increased likelihood of cross-reactivity.
Whole cells, exosomes, nucleotides, or other chemical components can also be used for antigen production. A number of computational tools exist that can help in the design of new antigens.
Tip: When validating your immunogen, it’s important to blast the peptide sequence against the UniProt database to see whether it has affinity to its stated target only.
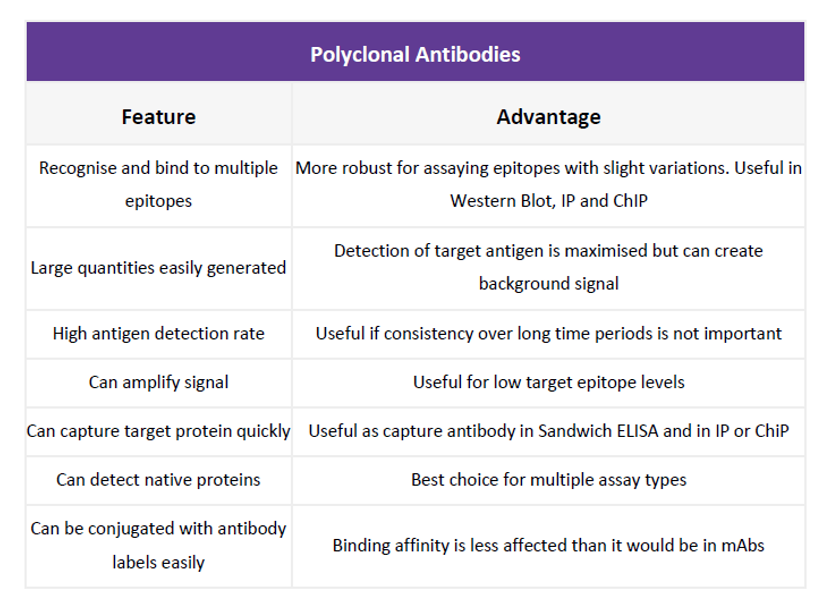
Choosing the best primary: monoclonal vs. polyclonal
The right primary antibody for your assay is ultimately dictated by your end research goals. Monoclonal antibodies are specific to one antigen only, whilst polyclonal antibodies bind to multiple epitopes. For that reason, polyclonals are much more robust in the detection of epitopes prone to variation or present in low quantities, yet non-specific binding is more likely to occur. This is a common source of error on western blots, making it tricky to distinguish the band that correlates to your protein of interest, but can be avoided by carrying out peptide blocking: incubating with excess peptide that corresponds to the recognized sequence. Antibody/peptide matching pairs can be purchased together for this purpose.
The biggest successes in therapy have been driven by monoclonal antibodies; their specificity for a single epitope reduces disease-only reactivity and normal cell cross-reactivity.
Selecting your secondaries
Secondary antibodies improve detection accuracy, amplify signal and provide wavelength flexibility. From target species, host species, Ig specificity, whole or fragmented, conjugated or non-conjugated, clonality, and application type, there are multiple factors to consider when selecting your secondaries for optimal results.
Discover top tips and considerations for selecting your secondaries, in this white paper >>
Antibody applications: Top tips for assay optimization
One important point to consider is the availability of the epitope for binding, and how this might change for each assay. For example, the conditions of the western blot workflow can alter the structural conformation of the protein, and therefore lessen its binding affinity. Validation is therefore key — where possible, Biorbyt validates its antibodies in mouse, rat and human paraffin-embedded sections for western blotting and immunohistochemistry. The Biorbyt team has helpfully put together some expert hints and tips for optimizing your antibody-based assays in the downloadable white papers below:
- Western Blotting: Tips and Tricks
- Immunohistochemistry: 9 Steps to Success
- ELISA: Optimizing Your Results
