Elucidating the Molecular Mechanisms of the Eye Lens: New Hope for Cataract Treatment
Research uncovers new roles of Eph and ephrin signaling in lens health
18 Jul 2019

Cataracts are the leading cause of blindness worldwide, however the cellular and molecular mechanisms of this condition are still not well understood. SelectScience® speaks to Dr. Catherine Cheng, Assistant Professor at Indiana University School of Optometry, to learn how she is elucidating the molecular mechanisms that underlie age-related eye lens defects, namely cataracts and presbyopia, and how water purification technology from ELGA LabWater helps achieve accurate results for the cellular and molecular experiments performed by the lab.
Tell us about your research.
My research interests focus on the lens inside of your eye. The lens is a transparent organ in the front of the eye that fine focuses light onto the back of the eye so you can see a clear image. I study two age-related lens defects; one is cataracts, or any opacity in your lens. You may know somebody who is older who has had cataract surgery to remove the cloudy lens inside their eyes and the natural lens is replaced by a plastic artificial lens. Currently, surgery is the only treatment for cataracts, and while this procedure is very commonly performed in the U.S., lack of access to a qualified surgeon in other parts of the world means that cataracts will remain the leading cause of blindness in the world. I study lens cell biology to understand exactly how cataracts occur and hope to utilize that knowledge to design new non-surgical treatments.
The second age-related disease that I study is presbyopia. This is the inability of your lens to change shape so that you can see up close. In a young person, the lens will change shape so you can focus on both near and far objects, and the focus point will change almost instantaneously. However, your lens keeps growing throughout your lifetime so it gets really big and really stiff and can no longer change shape so that you can see near-distance objects clearly. As a result, you need reading glasses to compensate for that. Currently, we don't understand the cellular and molecular mechanisms for this aging process.
What experiments do you need to perform in the lab day-to-day?
We routinely perform standard cell and molecular biology techniques, including cell culture, PCR, immunostaining and protein gels. Another big part of my research is microscopy: I use super-resolution confocal microscopy to look at the detailed structures of lens cells, and I do live lens tissue imaging in order to avoid fixation and tissue handling artifacts.
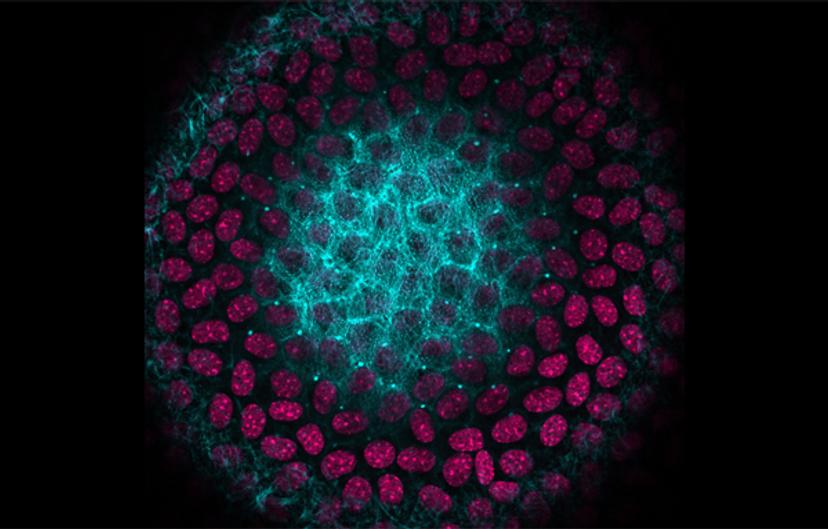
What are you working on right now and what are the implications of your work?
My current project is looking at Eph-ephrin bidirectional signaling in the lens. Eph are receptors on the cell surface; they're the largest class of receptor tyrosine kinases, and they bind to a class of ligands known as ephrins.
All the cells in your body express a complement of different Eph receptors and ephrin ligands, and the important distinction of this signaling pathway is that it's bidirectional. Usually, in a cell, a ligand binds to a surface receptor and the cell that has the receptor undergoes some sort of signaling process. But, in this case, when an Eph receptor binds to an ephrin ligand on a neighboring cell, both the cell with the receptor and the cell with a ligand undergo signaling. Thus, this induces bidirectional signaling.
In the lens, we know that one of the receptors, EphA2, and one of the ligands, ephrin-A5, are required for lens transparency. There are human and mouse studies, including my own, that show the disruption of Eph-ephrin signaling leads to cataracts. My lab is using mouse models to study how cataracts occur when you have dysfunction of the Eph-ephrin signaling system. We want to understand which receptors are utilizing the lens and which are their partner ligands. My work recently showed that EphA2 and ephrin-A5 are not a receptor-ligand pair in lens epithelial cells.
While Eph-ephrin signaling is less studied in the eye, this important signaling pathway is very well studied in other parts of the body. Dysregulation of Eph-ephrin signaling has been linked to neurological diseases, as well as cancer. Lots of labs are developing small molecules to try to disrupt or modulate this signaling pathway. I hope to identify the receptors and ligands that play a key role in maintaining lens health. The long-term goal would be to utilize small molecules targeting Ephs or ephrins, which are already being developed for other diseases, on the lens to prevent or delay either cataracts or presbyopia.
How do impurities in water affect your research?

We make lots of specialized buffers and solutions in the lab. The pH and osmotic balance of our solutions is especially critical, because the lens is sensitive to small changes that may lead to abnormal cellular changes. Any impurities in the water, ions, particles or biological contaminants may skew our results, making them non-reproducible in other people's labs. We don't want an impurity in our water to be causing an unusual result in our studies, and we definitely don't want that to be a false positive or a false negative. It’s really important, because we need to have confidence in our reagents.
Your lab uses the ELGA LabWater flex 2 water purification system, what made you choose this system and what benefits has it had for your lab?
When I was still a staff scientist at the Scripps Research Institute, I was recommended the ELGA water system by a sales rep. I had never heard of this brand before. From my perspective as a user, the ELGA system had quality consumables at a reasonable price. Another great thing is that the ELGA system was a good fit in terms of our daily usage needs. I really appreciated that the sales rep said: 'Look, you just need the Flex 2 system — it is good enough for what you need on a daily basis.' They didn't try to upsell me, and I appreciate that with the consumables, you can use them until they fail – there isn’t an artificial clock. In general, I think their consumables last a reasonable amount of time, basically 12 to 18 months for the filters, and about the same for the UV lamp, and that's what sold me on the ELGA system. In fact, I just purchased the same system for my new lab here at Indiana University. We had it installed around a month ago, and we are really happy with it.
What are your research findings so far about the Eph-ephrin relationship and what will you be working on next?
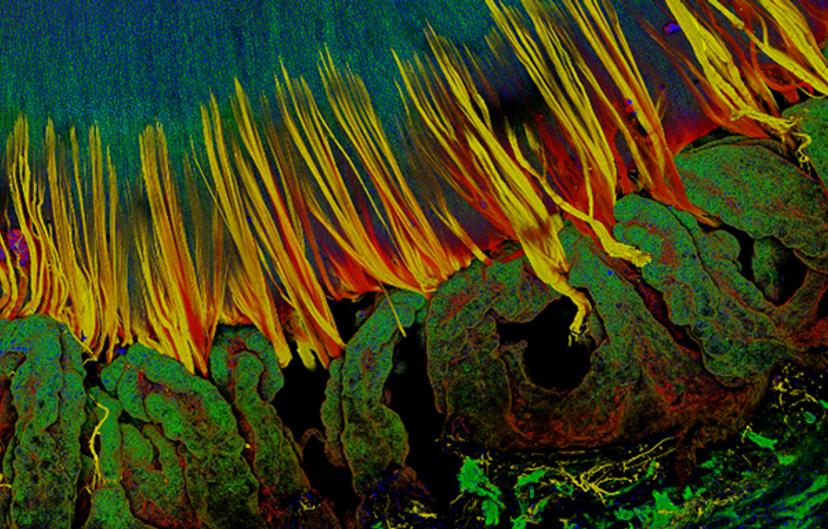
The lens fiber cells are hexagon in shape – they're packed tightly into the lens to minimize intracellular space and light scattering. Light will pass through and be focused instead of being scattered into your eyes, enabling you to see a clear image. We didn't know how this hexagonal packing occurred or what signals drive this hexagonal packing. It turns out EphA2 is required for this important cell packing process. When EphA2 is deleted from lens cells, the cells are no longer all hexagon in shape — you get pentagons and heptagons and other shapes. These abnormal cells are misaligned and mispacked. We didn’t know the mechanism for lens cell packing until we discovered this defect in lenses lacking EphA2.
Another thing that we've recently showed, using genetics, is that EphA2 and ephrin-A5 don't interact in the lens epithelial cells. The lens has epithelial cells and fiber cells. In the epithelial cell population, ephrin-A5 is responsible for one subtype of epithelial cells, versus EphA2, which is responsible for another subtype. We're now on the hunt for the actual ligand and receptor that bind to each, respectively. We aim to unlock the universe of Ephs and ephrins in the lens.
Currently, we are working on understanding the role of EphA2 in lens fiber cell reorganization and compaction as they mature and age.
What do you see for the future of your research and its impact on human health?
The ultimate goal is to discover the mechanism for age-related lens cell changes and be able to find a drug target for preventing, delaying and/or treating cataracts or presbyopia.
In the short term, I'd like to really understand how Eph-ephrin signaling works in the lens. Since mutations and polymorphisms in EphA2 and ephrin-A5 affect human patients, we hope that by understanding the signals required for normal lens homeostasis and transparency, we can test small molecules that disrupt Eph-ephrin signaling to prevent or delay cataracts, first in mouse models, and eventually, in human patients.
The long-term goal is to really understand which cellular and molecular mechanisms are in place to keep the lens healthy. Some people never develop cataracts; we want to understand what protective mechanisms are in place in those individuals. We know a lot about the morphology of lens cells, but we don't really know how the cells develop these very complicated interdigitations between one another. We aim to create new methods to study the lens and elucidate the mechanisms for lens health and disease.
Learn more about the Dr. Catherine Cheng’s work and the PURELAB® flex 2 by ELGA LabWater.

