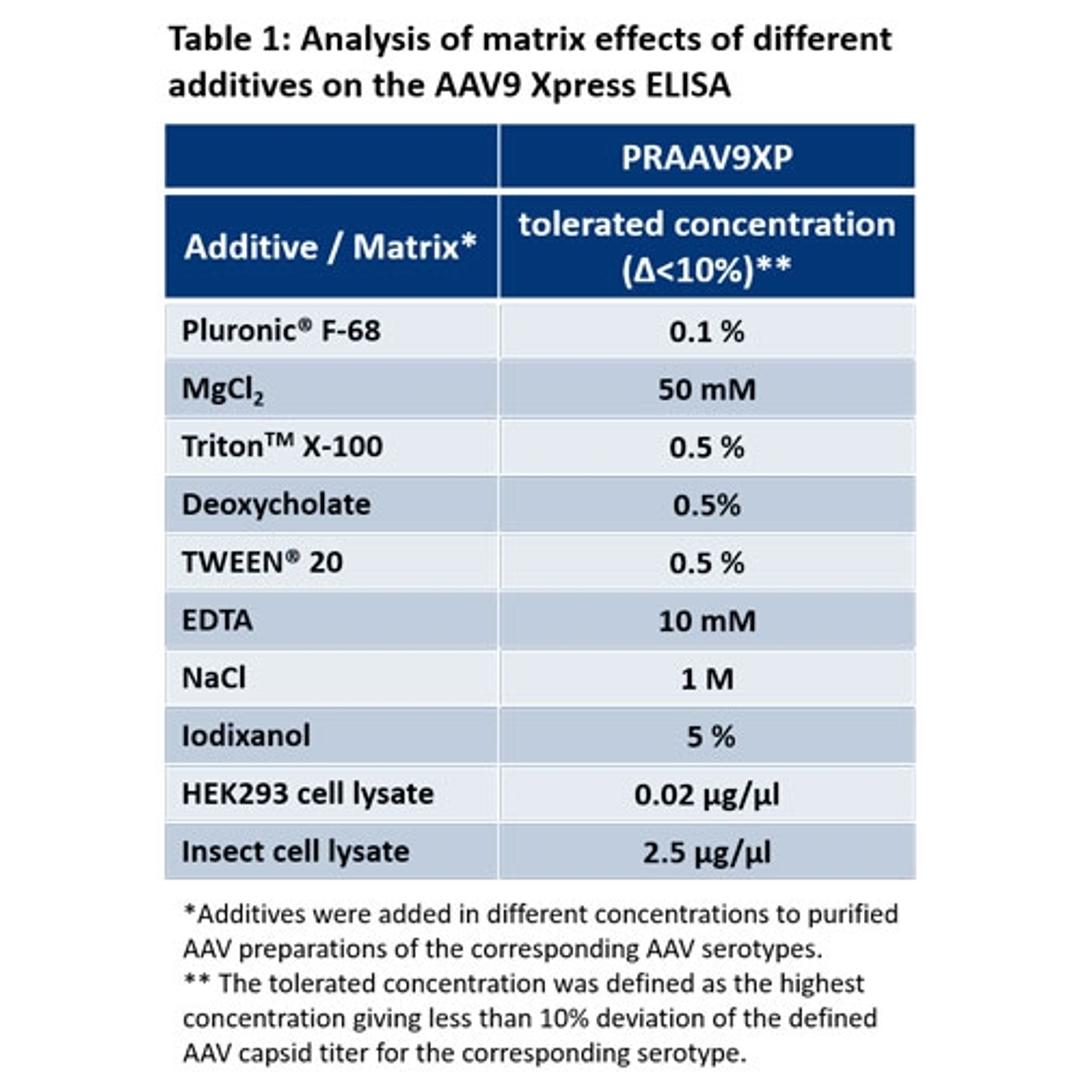
AAV9 Xpress ELISA
Fast, easy and reliable AAV9 titer determination: - get results in less than 2 hours and increase your daily sample throughput - easy workflow & process integration - same accuracy and reproducibility as the standard AAV9 Titration ELISA. Unique microtiter plate enzyme immunoassay for the quantitation of intact filled and empty AAV9 capsids of human Adeno-associated Virus 9. The capture antibody detects a conformat…

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Fast, easy and reliable AAV9 titer determination with PROGEN's AAV9 Xpress ELISA:
- assay time was reduced to less than 2 hours by adjustment of the kit components and the protocol of our standard AAV9 Titration ELISA
- seamless and risk-free transition from our standard AAV9 Titration ELISAs (PRAAV9) to the Xpress version since the composition of the kit’s core characteristics are not changed
- same accuracy and reproducibility as the standard AAV9 Titration ELISAs
Adeno-associated viruses (AAV) are nonpathogenic ssDNA viruses, which are a subject of intense studies as viral vectors for gene therapy. The virus transduces a variety of dividing and nondividing cells showing long-term gene expression with low cellular immune response. AAV has been used in several clinical trials (e.g. FIX, CFTR, Parkinson's, Canavan disease) showing no serious vector-related adverse effects. AAV9 is especially useful for applications in the CNS.
Methods for the characterization of AAV preparations currently include titration ELISA, real-time PCR, DNA dot blot, determination of transducing units, infectious center assay, SDS-PAGE or electron microscopy.
AAV preparations contain up to 10fold more empty virus capsids than filled, therapeutically active virus particles. Since both full and empty particles can induce an immune reaction, it is crucial for in in vivo appilcations to determine the complete particle titer.
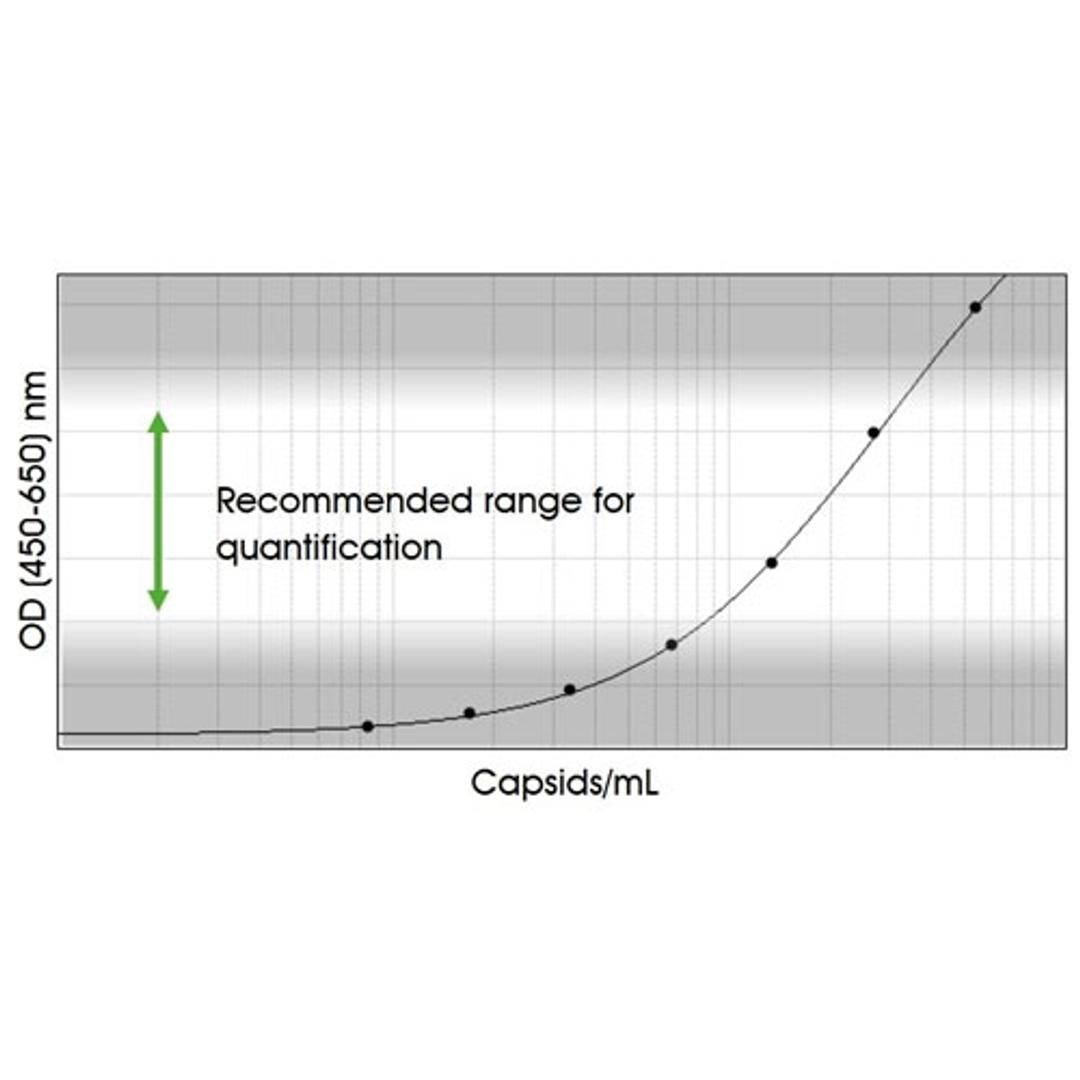
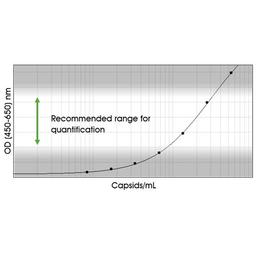
Immunotitration by PROGEN's AAV9 Titration ELISA offers a fast, sensitive and reproducible method for titration of intact AAV9 wt virions, AAV9 recombinant virions or assembled and intact empty AAV9 capsids.
The ELISA principle:
The assay is based on the sandwich ELISA technique where a monoclonal antibody (mab) specific for a conformational epitope on assembled AAV capsids is coated onto the plate and is used to capture AAV particles from the specimen.
The detection of captured AAV particles is a two-step process.
A biotin-conjugated mab is bound to the captured AAV particles.
A streptavidin peroxidase conjugate reacts with the biotin molecules. The addition of the substrate results in a color reaction which is proportional to the amount of specifically bound viral particles.
Comparison of AAV Quantification Methods:
Each of the commonly used quantification methods has its pros and cons:
- qPCR is widely used, but suffers from several issues such as sample preparation, primer design, or PCR efficiency that can lead to high inter-laboratory variation of results.
- Digital droplet PCR methods overcome some of the limitations of qPCR. However, variations between labs can still occur due to different sample processing protocols.
- Dot blot is a simple and quantitative method, if reliable reference material is used. However, it suffers from the limited linearity and dynamic range of western blotting in general.
Given the practical drawbacks of the aforementioned techniques, a conventional sandwich ELISA currently appears to be superior in terms of inter- and intralaboratory variation as well as ease of use. Therefore, it represents the best format for reliable and reproducible quantification of total rAVV capsid titers.
Use of shuffled/mutated AAV:
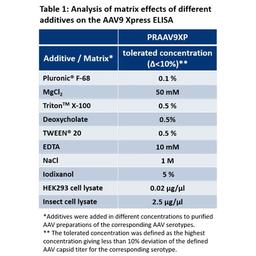
The recognition of shuffled/mutated AAV vectors depend on the specific capsid region which is affected by the shuffling/mutation. The capture antibodies used for PROGEN’s AAV ELISAs bind specific and, in some cases, well defined conformational epitopes. These epitopes are generated by the capsid assembly of the corresponding AAV serotypes. A first indication that the ELISA might recognize your shuffled/mutated AAV vector is the presence of the antibody-binding epitope. However, changes in the protein sequences of the capsid proteins might also influence conformation of the proteins, hence the conformation of the epitopes presented on the AAV capsid. This might influence binding affinity of the antibody and affect determination of the titer based on the (non-shuffled) Kit Control provided with the AAV ELISA kit. Since these properties strongly depend on the specific shuffling/mutation performed, PROGEN cannot guarantee successful and precise quantification of your shuffled/mutated AAV vector. Even if the antibody binding epitopes are still present on your shuffled/mutated AAV capsid, your assay needs to be tested and optimized for your specific AAV vector.
PROGEN highly recommends the production and calibration of a suitable (shuffled/mutated) Kit Control, to ensure reliable titer determination of your individually shuffled/mutated AAV vector with PROGEN ELISA kits.
Limited Use Label License: Research Use OnlyProduct is exclusively licensed to PROGEN Biotechnik GmbH. The use of these products for the development, manufacturing and sale of secondary products/derivativeswhich are based on the purchased products and/or which include the purchased product require a royalty based sub-license agreement.