Bioprocessing Salt Active Nucleases - High Salt Conditions
Salt Active Nuclease High Quality (SAN HQ) is a Bioprocessing Grade nuclease available in GMP grade developed as the most efficient solution for removal of both single and double stranded DNA and RNA at high salt conditions(400–700 mM). It excels in DNA removal for robust viral vectors like Adenovirus and AAV, ensuring superior product purity while improving DSP. Ideal for gene therapy and vaccine manufacturing. Triton free av…
SAN HQ and SAN HQ GMP: The Ultimate Solution for DNA Clearance in Adenovirus & AAV Bioprocessing
Optimized for high-salt environments, SAN HQ ensures unparalleled DNA removal without compromising the integrity of robust viral vectors.
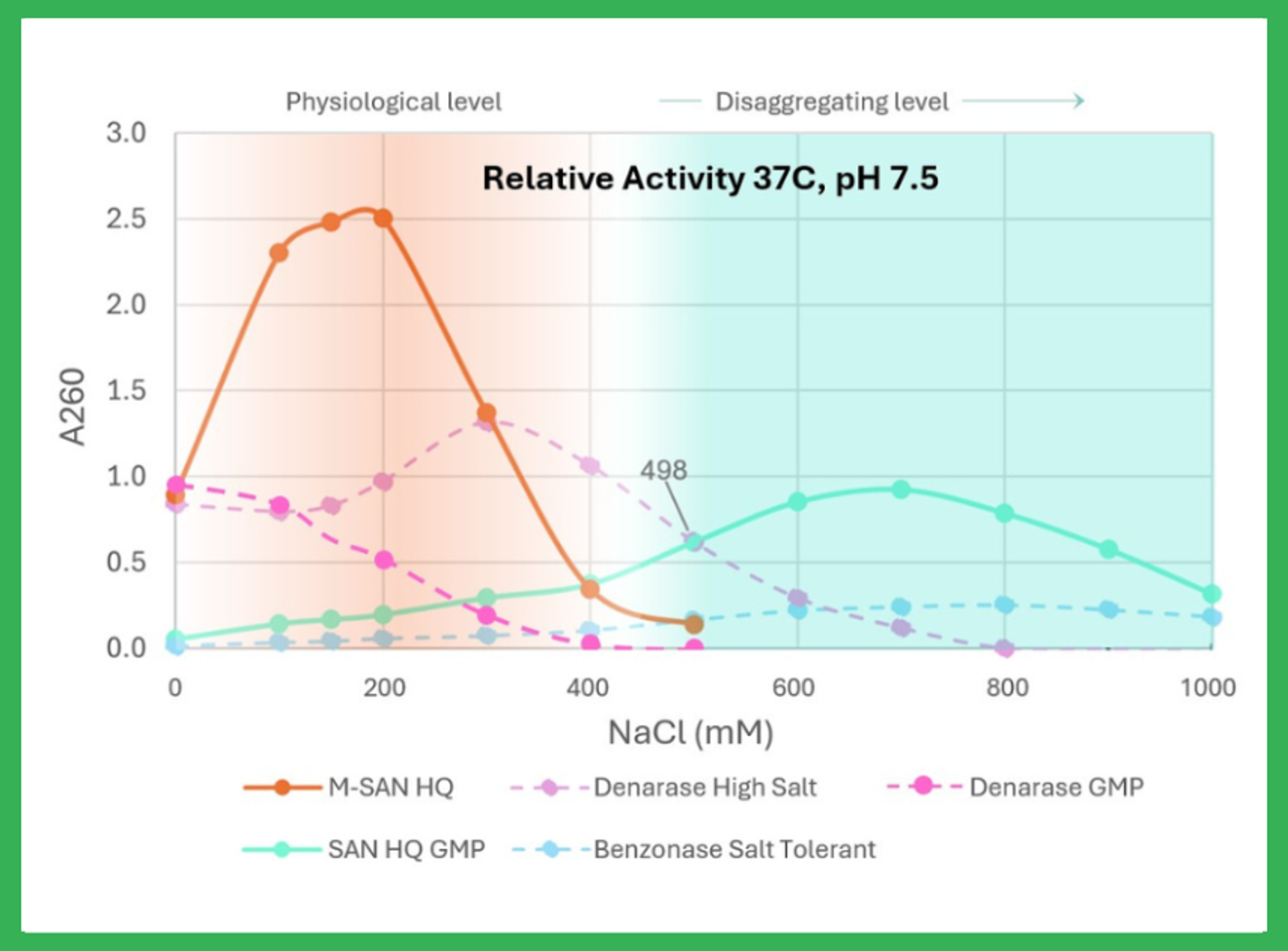
Salt Active Nuclease High Quality (SAN HQ) and SAN HQ GMP are Bioprocessing Grade nucleases developed as the most efficient solution for removal of nucleic acids, at high salt conditions. This nonspecific endonuclease has peak activity at salt concentrations between 400 – 700 mM.
The Big Picture
Reducing residual DNA in lysate is not a procedural step; it's a regulatory must-do that directly impacts product quality and safety.
Zoom In
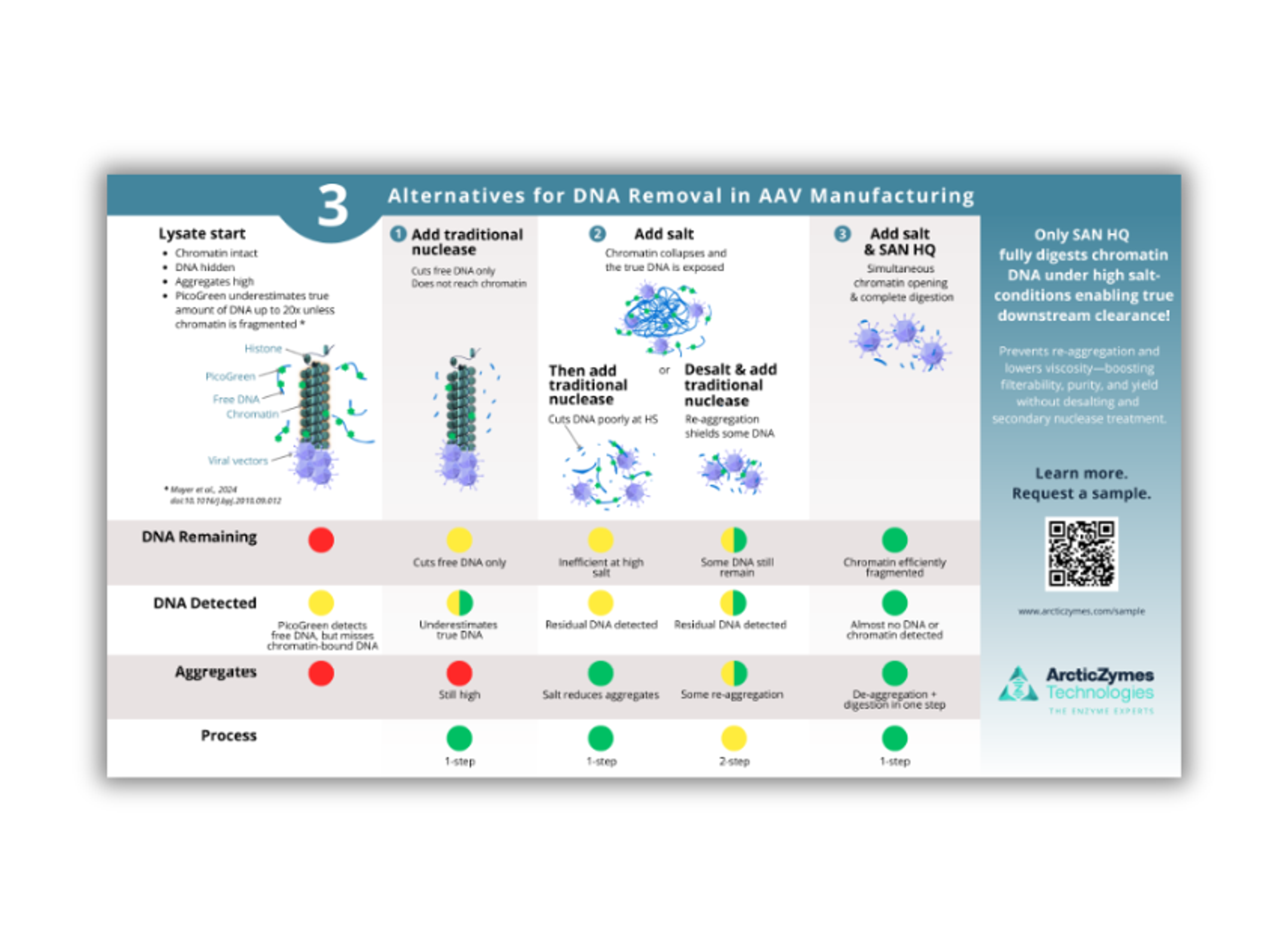
SAN HQ effectively degrades Chromatin DNA to sizes that can be fully trapped and removed during purification, ensuring compliance with strict regulatory criteria.
Why It Matters
Effective DNA clearance not only ensures AAV vector purity but also minimizes patient risk. It's not just about compliance; it's about advancing the quality of gene therapy.
The Bottom Line
DNA clearance is non-negotiable for compliance, safety, and market success.
Key benefits of SAN HQ:
- Optimized Residual DNA Removal: Efficiently degrades residual DNA in high-salt conditions, meeting stringent quality and regulatory requirements.
- Enhanced AAV Vector Purification: Improves the purification process for adeno-associated viral vectors, boosting quality, yield, and viral stability.
- Streamlined Workflow: Eliminates the need for desalting stages, facilitating high-throughput processes and simplifying bioprocessing protocols.
- Economized Enzyme Usage: Reduces excess enzyme requirements and additional adjustments, leading to significant cost savings.
- Reliability and Efficiency: Provides consistent performance in high-salt conditions, minimizing disruptions and ensuring efficient host cell lysis.
- Broader Applicability: Versatile for use in various viral vector systems, expanding research and production capabilities.
- Product Safety and Purity: Enhances safety and purity by minimizing contaminating host cell DNA, ensuring high-quality therapeutic viral vectors.
Applications:
- Purification of biologics from residual nucleic acids in biopharma manufacturing
- Purification of recombinant proteins and enzymes for research and diagnostic use
- Removal of unwanted nucleic acids contamination in molecular biology reagents in challenging conditions
- Reduction of viscosity in biological samples during production and automation
- Vaccine manufacturing and viral vector preparation
- DNA removal in high-salt lysates