ProBioGen Pharmaceutical Cell Line Development Service
Stable, High-Yield Mammalian Producer Cell Lines. ProBioGen's in-house cell line engineering platform enables them to provide our clients with pharmaceutical producer clones that exceed the limits of the current industrial standard, while still supporting a royalty-free fee-for-service business model.ProBioGen have built a fully integrated technology platform that encompasses the key actuating variables: Host Cell Line, Vecto…

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Stable, High-Yield Mammalian Producer Cell Lines.
ProBioGen's in-house cell line engineering platform enables them to provide our clients with pharmaceutical producer clones that exceed the limits of the current industrial standard, while still supporting a royalty-free fee-for-service business model.
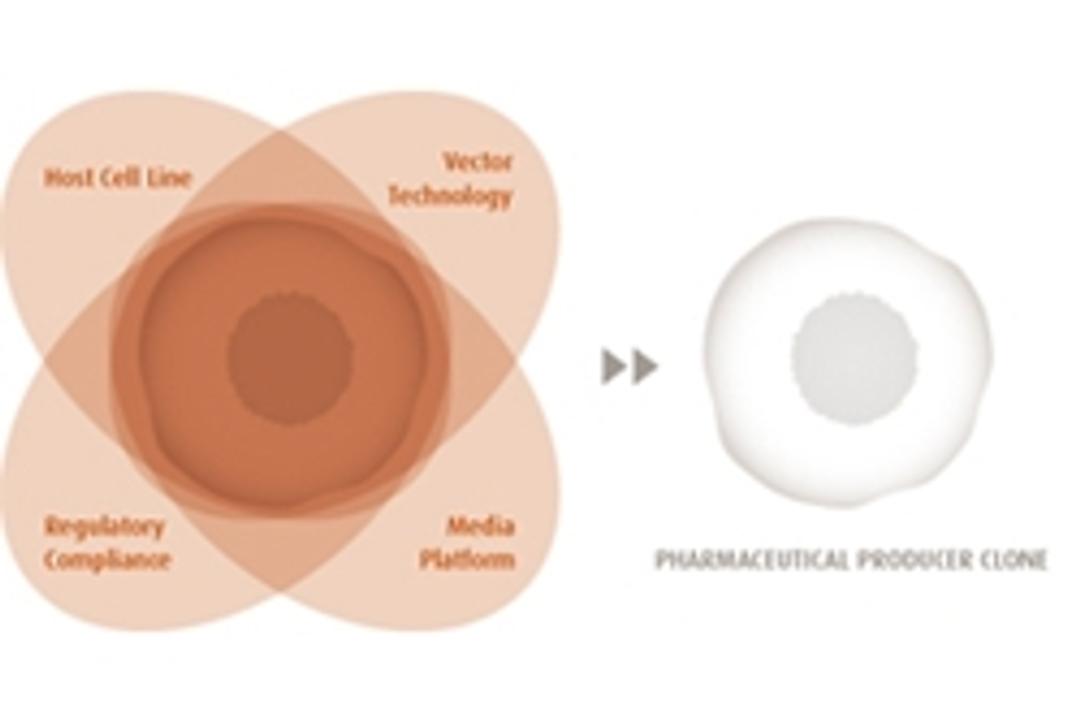
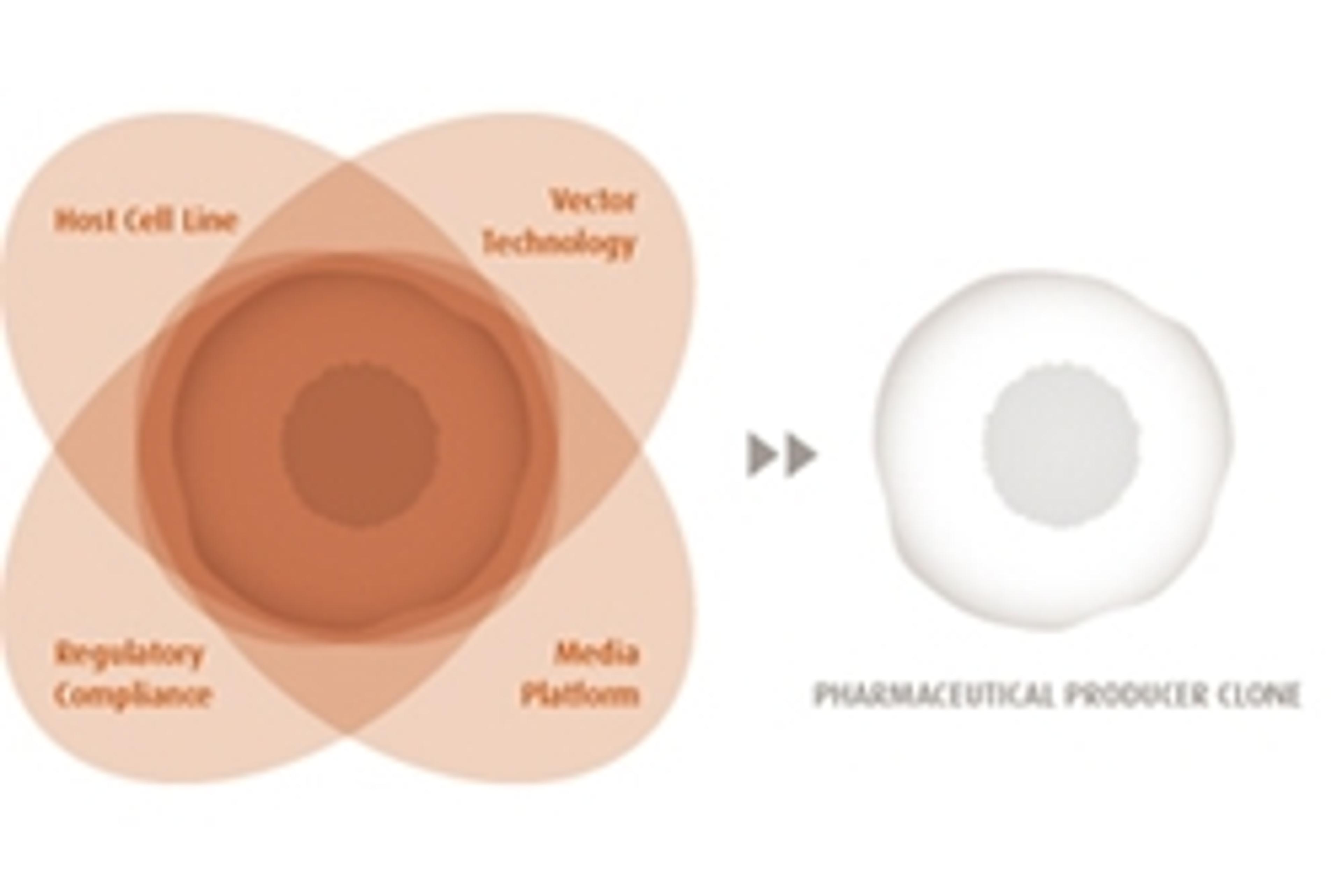
ProBioGen have built a fully integrated technology platform that encompasses the key actuating variables: Host Cell Line, Vector Technology and clone-adapted Media Platform.
Key Advantages of ProBioGens Production Cell Lines:
- Expression Yield - Achieve predictable and stable high mAb-expression levels in the 1–4 g/L range (non-optimized fed batch). ProBioGen antibody expression cassettes thus employ fine-tuned promoters and other genetic regulatory elements to support a perfectly balanced and synchronized subunit expression.
- Upstream Performance and Scalability - Key upstream performance parameters (qp, IVCD, titer) remain consistent during upscale. The value of a candidate clone typically becomes apparent once it is used in a regulated fermentation process at process development scale. This "Bioreactor Performance Analysis" (BPA) is of high predictive value and assures maintenance of clone performance up to large-scale fermenters.
- Clone Stability - ProBioGen's producer clones maintain a consistently stable and high transgene expression level throughout the duration of the manufacturing time window.
- Regulatory Acceptance - The documentation for our production clones addresses all aspects covered in the relevant guidelines (ICH-Harmonized Tripartite Guidelines, EMA/FDA Points to Consider and Notes for Guidance) and contains submission-ready data to be included in the "body of data" section 3.2 of Module 3, CTD.