Search results for "slas europe 2018"
Selected Filters:
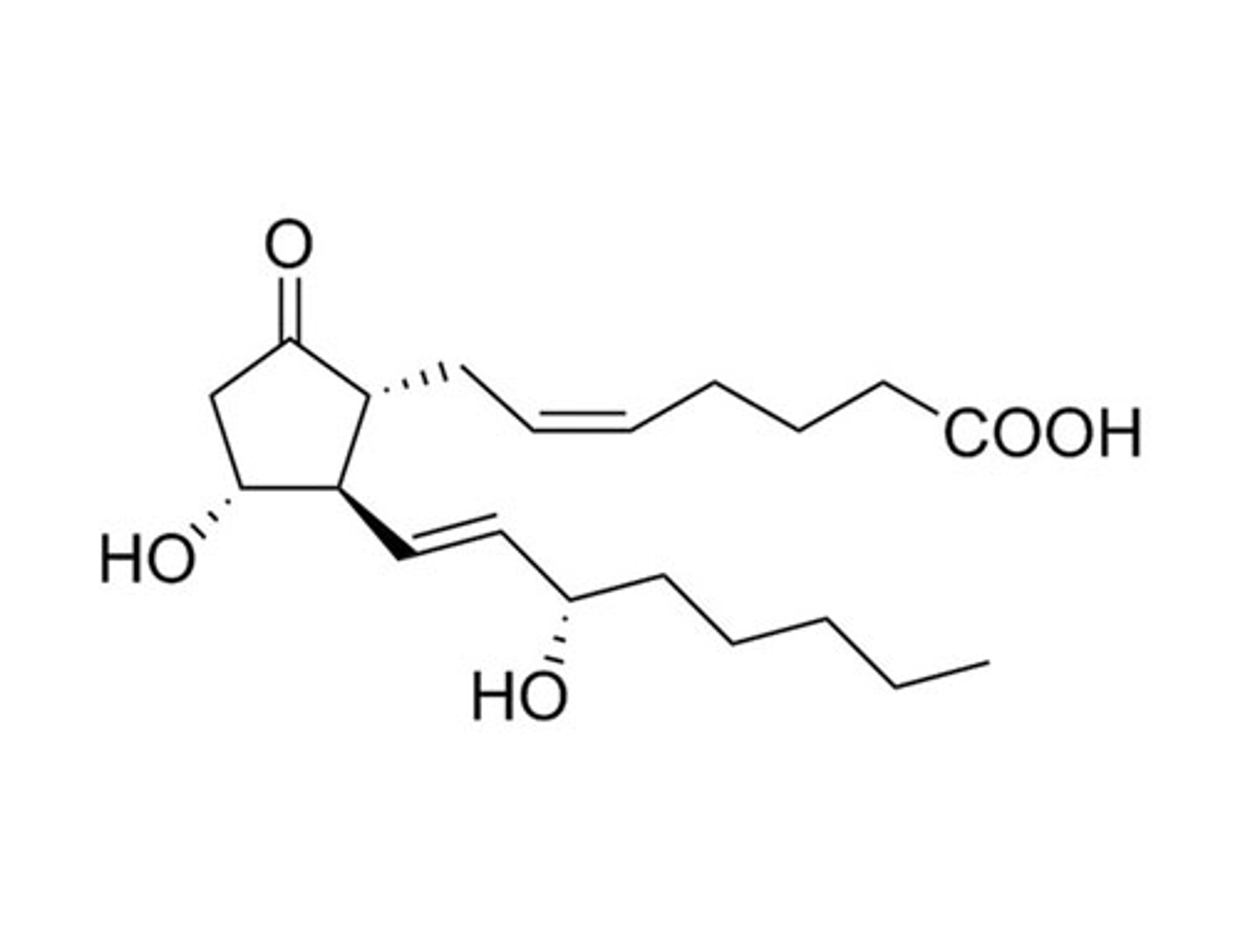
Prostaglandin E2
STEMCELL Technologies Inc.Prostanoid pathway activator; Activates prostaglandin receptors EP1, EP2, EP3 and EP4
Control Temperature Precisely with DYNEO
Discover the new temperature control technology for demanding applications
Research explores new DSC-thermomicroscopy approach to reach new depths in material property analysis
Researchers at the University of Huddersfield have combined differential scanning calorimetry (DSC) with thermomicroscopy to reveal detailed energy changes in specific materials
Integrated DNA technologies launches complete NGS library preparation solution for monkeypox
Leading genomics solutions provider debuts complete NGS library preparation to support the identification of novel monkeypox variants in response to growing researcher demands for viral surveillance solutions
Rigaku’s analysis of the asteroid Ryugu by WDXRF and thermal analysis will be invaluable for future research projects
The analysis hopes to unlock the secrets behind the formation of our solar system
bioMérieux receives De Novo FDA Authorization for its BIOFIRE Joint Infection (JI) Panel
The BIOFIRE solution is a U.S. FDA-cleared and CE-marked closed multiplex PCR and fully automated system that integrates sample preparation, amplification, and detection
‘Scientific communication is essential, both between scientists but also to the general public’
Alexandre Lucas discusses the need for science communication, and how they enable him to bring new ideas to the table
Teledyne ISCO release new line of syringe pumps
The new SyriXus line of high-performance syringe pumps is now available for ordering
Food scientists create zinc index for human body
‘Thank you for placing your trust in SelectScience’
SelectScience CEO Kerry Parker shares our 2021 Queen’s Award success with scientists and manufacturer partners around the world
Montgomery County Police Department Crime Lab: The latest forensic laboratory in U.S. to use STRmix
STRmix forensic software is highly effective in interpreting complex DNA evidence
ENPICOM and Viroclinics-DDL receive joint MIT Zuid subsidy to accelerate and improve antibody and vaccine discovery and development
The partnership combines Viroclinics-DDL’s diagnostic and high-throughput sequencing experience with ENPICOM’s expertise in bioinformatics and immunomics
Promega's new workflow allows COVID-19 testing labs to skip RNA extraction
XpressAmp Direct Amplification Reagents facilitate RNA extraction-free sample preparation that is automation-friendly
Gas chromatography high-resolution mass spectrometer offers new standard of performance for research laboratories
The Thermo Scientific Orbitrap Exploris GC 240 Mass Spectrometer promises high data quality and versatility to accelerate scientific discovery for academic, industry research, government and omics laboratories
Expand your research skills with our latest expert webinars
Discover the new techniques labs are using to meet strict weighing compliance requirements and check out our series of on-demand webinars
MicroPro in the Lab: Avidien announces winner of microPro giveaway contest
For their prize, Dr. Lieberman and her team have chosen to receive the upcoming low volume model, the microPro 25
New formula for molecular probes
The new formula incorporates genetic manipulation of the optical refractive index of live cell components leading to their use as molecular probes demonstrated for the first time
Developing a 10-minute fingerprint test for COVID-19
A portable and non-invasive, fingerprint-based method, ideal for on-site testing in care homes and the workplace