Separating polar analyte mixtures: Benefits of a new zwitterionic HILIC chemistry
Watch this on-demand webinar to discover a new zwitterionic HILIC stationary phase that addresses the need for improved pH stability
20 Jan 2022

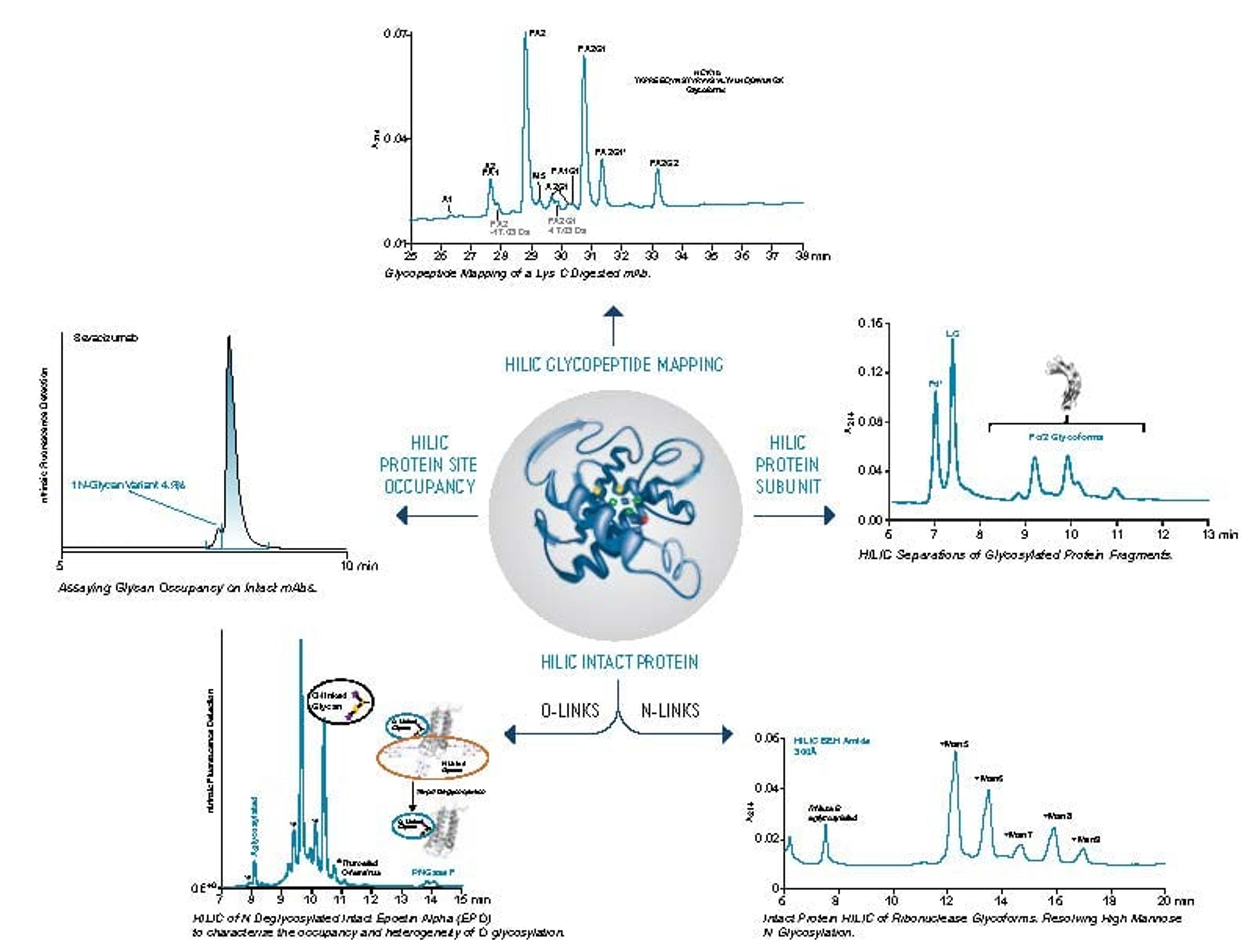
Hydrophilic interaction chromatography (HILIC) is one of the most effective approaches for separating polar analyte mixtures. Of the stationary phases used for HILIC, zwitterionic chemistries have been among the most popular due to their good retention for a wide range of polar compounds.
In this SelectScience webinar, now available on demand, Jonathan Turner, product marketing manager at Waters, describes a new zwitterionic HILIC stationary phase based on ethylene-bridged hybrid (BEH) particles that address needs for improved pH stability while providing an alternative selectivity to existing HILIC chemistries. Turner also highlights how using column hardware with inert surfaces can mitigate unwanted interactions between polar analytes that are known to interact with metal surfaces.
Watch on demandRead on for highlights from the Q&A discussion and register now to watch the webinar on-demand
What are the advantages of using HILIC columns for polar analytes compared to derivatization methods?
JT: I don't like to do extra steps in my workflow such as derivatization. It adds another layer of complexity and can introduce additional variability. When you talk about derivatization versus underivatized, I would always lean towards underivatized where possible if it's meeting your method goals. I do understand however that for some workflows, derivatization is the only process that will get you to the goals that you're looking to achieve.
How do you condition a new HILIC amide or zwitterionic HILIC column when first opened?
JT: How we typically recommend conditioning a column, and we have care and use guidelines for all our stationary phases, is to first flush the column. We typically recommend a 50% water, 50% organic mobile phase for about 20 column volumes. And then put that column into the starting mobile phase conditions that you're going to run, typically higher organic greater than 80%, and let that run for about 50 column volumes. It takes a little bit of time to establish the aqueous layer on the surface of the column that’s needed for partitioning.
For organic acids, such as citric acid, oxaloacetate, formulated sugars, and nickel oxalate, would you recommend BEH amide or BEH zwitterionic HILIC chemistries?
JT: The BEH amide column, typically has good selectivity for a lot of these acidic compounds. But for things like isocitric, and citric acid, specifically, we see some good resolution between those two with the BEH HILIC columns. It depends on what the specific application goals are, as both will be applicable. If you're looking for a little bit more retention, the zwitterionic will give you greater retention versus the BEH amide.
Is it possible to measure very polar compounds such as sugars on this column?
JT: HILIC is ideal for polar compounds, the more polar it is, the better HILIC works. Sugars are broad classes of compounds. Reducing sugars are typically done with our BEH amide. There will be anomers present, which you need to typically collapse so our preferred HILIC column for that is our BEH amide. Waters has a lot of great application notes on separating carbohydrates and sugars with the amide stationary phase.
Have you got any tips to reduce the tailing that HILIC peaks normally come with?
JT: Often tailing typically comes down to some sort of secondary interaction that you don't want. When it comes to tailing, often what we find is that buffers can be very helpful in reducing the tailing. I know it's easier just to pipette in a little bit of formic acid or a little bit of ammonium hydroxide but what we've seen is a buffer, whether it's ammonium formate or ammonium acetate, can be helpful in 10 millimolar concentrations in providing increases in peak shape. These buffers are all MS friendly too.
If you use MaxPeak columns, should comparable PEEK to PEEK in-line stainless tubing or connections be used in the entire liquid chromatography system?
JT: The column has the highest surface area in the flow path and that's primarily due to the inlet and outlet frits that are inside the column itself. So, you'll see benefits from just using the column, with the MaxPeak Premier Column inline. But if you can swap out the tubing in the flow path from the LC and the tubing going into the detector, that will give you another level of increased performance.
To learn more about the new zwitterionic HILIC stationary phase, watch the full webinar here>>
SelectScience runs 10+ webinars a month across various scientific topics, discover more of our upcoming webinars>>