The pursuit of the perfect HPLC column: Reflecting on 50 years of innovation
Dr. Thomas H. Walter, of Waters Corporation, looks back at the trials, tribulations, and triumphs that paved the way to today’s advanced separation technologies
3 Jul 2023
The ideal HPLC column is highly reproducible, stable when exposed to a wide range of mobile phases and temperatures, and able to produce narrow and symmetrical peaks for all analytes of interest. Since the development of the first commercially available HPLC columns over 50 years ago, scientists have made substantial strides toward reaching these ideals, bolstered by steady improvements in the technologies used to synthesize stationary phases, fabricate column hardware, and pack columns.
As part of our 25th anniversary, 'Accelerating Science: The people and technologies changing the world' special feature, SelectScience® spoke with Dr. Thomas H. Walter, Corporate Fellow at Waters™ Corporation, to learn more about the key innovations that have driven improvement in HPLC column technology, how close we are to realizing the perfect column, and what he sees for the impact of advanced separation technologies on future scientific progress.
Dr. Walter joined Waters in 1987 after completing a Ph.D. in Chemistry at the University of Illinois at Urbana-Champaign. Throughout his distinguished career, he has co-invented multiple technologies and directed programs that led to the development of Waters’ most successful HPLC and UHPLC column families, including Symmetry™ Columns, Xterra™ Columns, XBridge™ Columns, XSelect™ Columns, ACQUITY™ Columns, and CORTECS™ Columns. In recognition of his long-term scientific achievements, Dr. Walter was inducted into the prestigious Jim Waters Society in 2017. More recently, he was announced as the 2023 recipient of the Uwe. D. Neue Award in Separation Science, a distinguished accolade created to commemorate the legacy of the late scientist Dr. Uwe D. Neue, a Waters Corporate Fellow.
A reproducibility revolution
High-performance LC columns were first introduced around 50 years ago, with Waters μBondapak™ Columns being among the first to use 10 μm silica particles and silane bonding to make relatively stable stationary phases. Since then, HPLC columns have progressed in several important aspects, primarily through changes to the particle size and the chemistry of the stationary phase.
In the early days of HPLC, the packing materials in columns such as µBondapak Columns were comprised of irregularly shaped particles. “It was the work of Dr. Uwe Neue and colleagues that took us from μBondapak Columns to Nova-Pak™ Columns, which was our flagship column when I joined the company,” explains Dr. Walter. “At the time, these columns were state of the art and represented the first time Waters manufactured silica particles. Spherical particles were a big step forward, as they could be packed better to deliver high efficiencies, and enabled the use of smaller (4 μm) particles. However, Nova-Pak Columns silica is made from raw materials that have significant levels of metal impurities such as sodium, aluminum, and iron, which has a negative impact on the way they perform.”
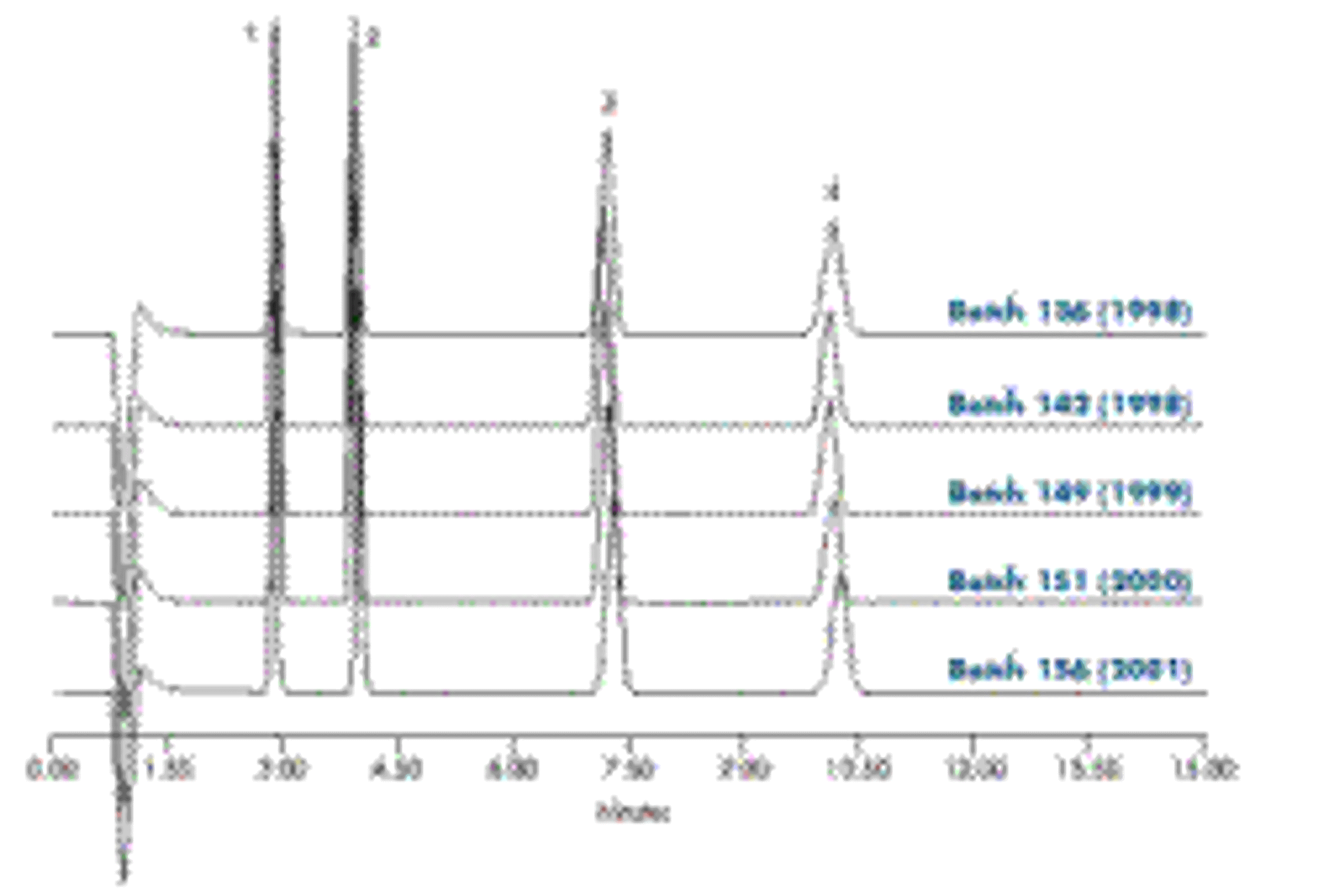
In the early 1990s, Waters sought to address this issue by synthesizing high-purity silica, improving bonding chemistries, and developing more tightly controlled manufacturing processes. These efforts culminated in the launch of Symmetry™ Columns in 1994, which established a new standard of reproducibility across the industry. “By tightly controlling all raw material and process variables, we were able to reduce batch-to-batch variation by about ten-fold compared to the original μBondapak Stationary Phases,” Dr. Walter enthuses. “Moreover, for the first time, quality control test results and specifications were reported in detailed certificates of analysis provided with every column.”
“Symmetry Columns were commercially successful and played a significant role in Waters' growth during the time when it became an independent company again,” he continues. “It marked a return to focusing on our core products and customers, those being reversed-phase columns for the small molecule pharmaceutical industry.”
Enter hybrid particles
Symmetry Columns marked a substantial improvement in performance and batch-to-batch reproducibility. However, the utilization of silica-based stationary phases posed a persistent challenge due to their limited usability above pH 8, beyond which particle dissolution becomes a significant issue, leading to a loss of column efficiency and poor peak shape. Organic polymers are alternative packing materials that address pH stability concerns, but they have their own drawbacks including low efficiency and poor mechanical strength.
To address these challenges, Waters sought inspiration from the materials science community, which had published numerous studies on hybrid materials that combine the advantageous properties of both organic and inorganic materials. “We had the fortune of having a material scientist (Zhiping Jiang) join the company who had a knowledge of hybrid materials,” explains Dr. Walter. “He made a prototype that could tolerate higher pH without taking on all the limitations of polymer-based columns, and this technology went on to be used in XTerra™ Columns, which were launched in 1999.”
XTerra Columns were also a commercial success, and marked a milestone because it restarted the Waters Chemistry group on a path of focusing on innovation.
Dr. Thomas H. Walter, Waters Corporation
XTerra Columns contained the first commercially available hybrid organic/inorganic stationary phases, which provided improved stability to alkaline mobile phases compared to silica-based materials as well as decreased silanol acidity. “XTerra Particles are synthesized from two monomers, one that forms SiO2 units and one that forms SiO1.5CH3 units – the presence of the latter units throughout the particle confers its special properties,” explains Dr. Walter. “XTerra Columns were also a commercial success, and marked a milestone because it restarted the Waters Chemistry group on a path of focusing on innovation.”
Delivering under pressure
XTerra Particles began Waters’ pursuit of smaller particle-size stationary phases, with 2.5 μm particles being used in short columns to enable faster separations. “These columns were designed to be used with conventional HPLC systems, at pressures less than 5000 psi,” explains Dr. Walter. “However, as we started to work with <2 μm particles and higher pressures in the early 2000s, we found that XTerra Particles did not have the mechanical strength to be packed at the pressures needed to make columns that were stable under these conditions.”
Contemplating the cause behind XTerra Particles’ insufficient strength, Dr. Walter surmised that the presence of SiO1.5CH3 units leads to a reduction in the average number of siloxane bonds per silicon atom – bonds that are essential to the mechanical stability of silica. While searching for a way to overcome this issue, he read a 1995 article in Chemical Reviews written by Professors Doug Loy and Ken Shea on bridged polysilsesquioxanes. These are hybrid materials that contain organic groups connected to two silicon atoms, for example with O1.5SiCH2CH2SiO1.5 units. Crucially, with this type of structure, the organic groups maintain the connectivity of the silica lattice. “I asked my colleague Ray Fisk to try making particles with this approach, and the early prototypes were promising, showing better alkaline stability than Xterra Particles,” says Dr. Walter. “As one of my first initiatives when I became the director of the Chemistry R&D organization in 2000, I started up a project to develop a stationary phase based on these ethylene-bridged hybrid (BEH™) Particles.”
As this work progressed, a major company project started that would culminate in the launch of ACQUITY™ UPLC Systems and Columns. "This was the highest risk project that I ever worked on, as success hinged on developing columns packed with <2 μm particles that not only gave the highest efficiencies per unit length that had been achieved in commercial columns but also had the mechanical robustness to tolerate pressures that were three times higher than what had previously been used in HPLC, up to 15,000 psi,” shares Dr. Walter. One of the first decisions was what particle to use for these columns. “Silica was the safe choice, but our highest performance silica particles were not robust enough mechanically when used at the highest pressures, so instead, I chose our BEH Particles, which were still being developed.”
To realize the theoretical efficiencies of an ultra-high-pressure system, the columns needed near-perfect packing and exceptional stability. “The packing element I was confident on, but the stability was a different animal,” shares Dr. Walter. “We had never tried to test column stability under those pressures before, and it turned out to be exceptionally challenging. Until we had done it, I wasn't sure we would be able to.”
Against the odds, Waters’ synthesis team, led by Kevin Wyndham, completed the development of 1.7 μm BEH Particles and provided the quantity needed for the launch of the ACQUITY UPLC System in 2004. The introduction of ACQUITY UPLC Systems not only changed Waters’ trajectory but that of the whole industry. The system brought unparalleled resolution and sensitivity, significantly decreased analysis times, and reduced solvent consumption compared to traditional HPLC methods. “As the adoption of UHPLC grew in the ensuing years, ACQUITY BEH Columns became our best-selling columns,” shares Dr. Walter. “We spent the next decade expanding our portfolio of ACQUITY BEH Columns, including chemistries for hydrophilic interaction chromatography (HILIC) and size-exclusion chromatography (SEC), as well as Ultraperformance Convergence Chromatography – a high-performance form of supercritical fluid chromatography. We also created larger particle size versions of these stationary phases, enabling them to be used on conventional HPLC systems, which are called XBridge™ BEH Columns.”
Modern ACQUITY UPLC Systems and Columns can now achieve about 30 times higher efficiency per unit time than μBondapak Columns on a conventional HPLC system.
Dr. Thomas H. Walter, Waters Corporation
Progress toward the ideal column
Over the past half a century, the innovations spearheaded by the team at Waters have revolutionized the reproducibility, speed, sensitivity, and resolution of chromatography. “Modern ACQUITY UPLC Systems and columns can now achieve about 30 times higher efficiency per unit time than μBondapak columns on a conventional HPLC system,” Dr. Walter enthuses. “Peak shapes have also greatly improved for a wide variety of analytes, with the challenges caused by residual silanol groups being effectively mitigated through the use of high-purity silicas, embedded polar group bonded phases, and hybrid organic/inorganic particles.”
Column stability has also been a focal point of innovation, with particular emphasis on addressing degradation mechanisms under both acidic and basic conditions. “The use of trifunctional bonding chemistries or sterically protected silanes has driven large improvements in stability to acidic mobile phases,” explains Dr. Walter. “Similarly, advancements in alkaline stability have been achieved by increasing bonding coverage or utilizing bidentate silanes, while the greatest improvements have resulted from employing hybrid organic/inorganic particles, particularly BEH Particles.”
Another pivotal area of improvement has been in column inertness, specifically regarding the column hardware. Traditional stainless steel columns are susceptible to binding of certain analytes, resulting in a myriad of issues ranging from peak broadening and tailing to variable peak areas and even complete loss of analyte signals. While alternative materials such as polyetheretherketone (PEEK) and polyethylene have been explored, these have their own issues, including decreased pressure tolerance and some solvent incompatibilities. In response to this challenge, Waters has introduced MaxPeak™ Premier Solutions, a new family of technologies that applies an inert barrier layer to metallic column hardware. “This technology significantly mitigates binding to metal surfaces without compromising the pressure tolerance of the columns, thus improving accuracy and reproducibility when separating analytes that interact with metal surfaces,” explains Dr. Walter. More recently, a hydrophilic version of hybrid surface technology has been developed to give improved SEC separations of monoclonal antibodies and related biopharmaceuticals.
Looking ahead
Separation science is an invaluable contributor to a variety of disciplines and is likely to remain so for the foreseeable future.
Dr. Thomas H. Walter, Waters Corporation
While significant progress has been made in the pursuit of the perfect HPLC column, new analytical challenges will continue to emerge as application areas evolve. “The challenges posed by novel drug modalities, food and environmental testing, omics, and other fields will continue to drive advancements in column technology,” asserts Dr. Walter. “For example, there will always be applications that drive a need for faster separations. These are likely to be in areas that require the analysis of a large number of samples, such as in personalized medicine and related biomedical studies.”
With current research in separation science predominantly focused on applying separation technologies to address specific problems, Dr. Walter encourages researchers interested in a career in separation science to specialize in a particular application area, such as biopharmaceutical characterization, biomedical research, omics, or food and environmental testing. “Separation science is an invaluable contributor to a variety of disciplines and is likely to remain so for the foreseeable future,” he says. “HPLC, being the most versatile method for analyzing both small and large molecule pharmaceuticals, as well as a wide range of essential biomolecules in human health and food quality studies, is unlikely to be replaced anytime soon,” he concludes.