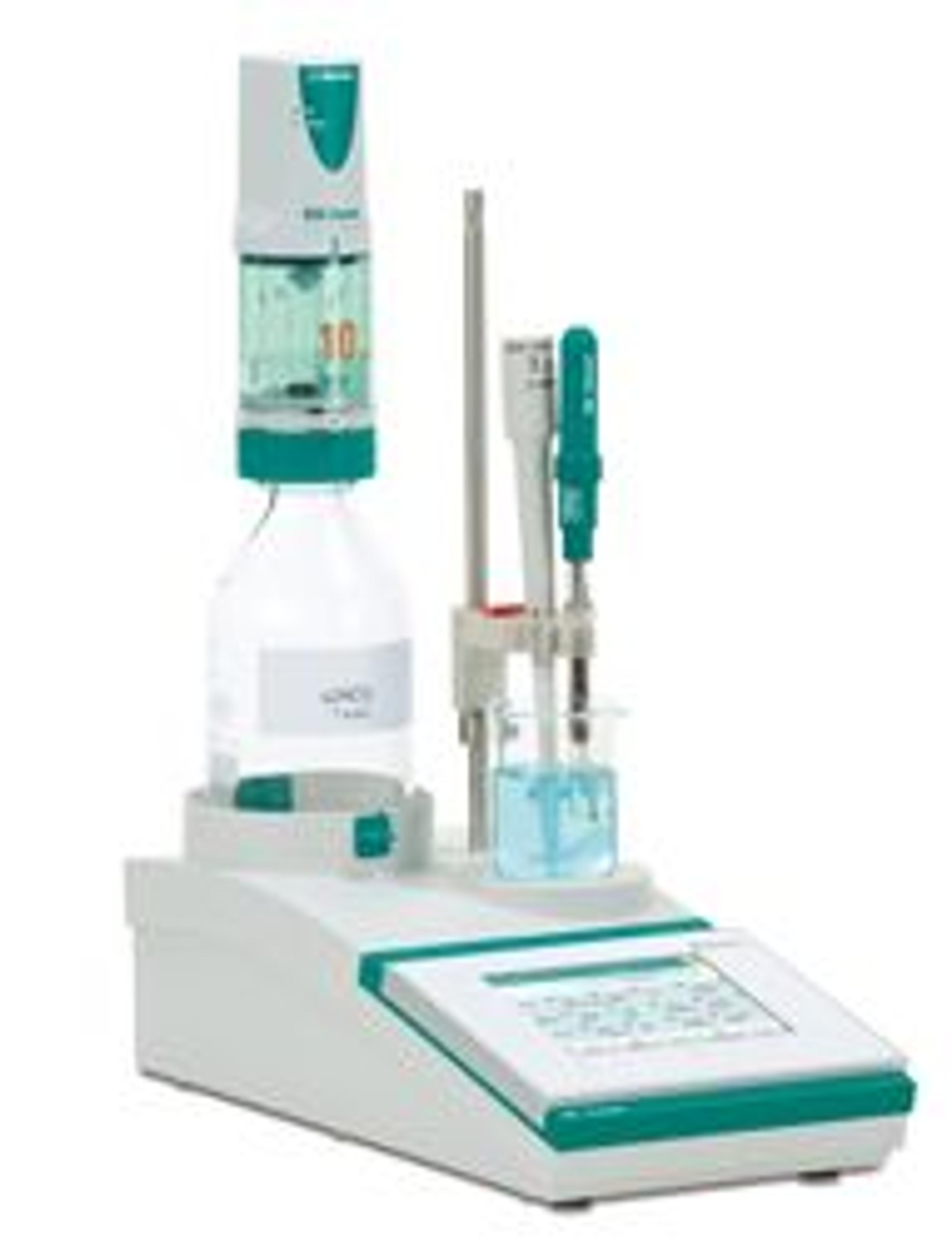
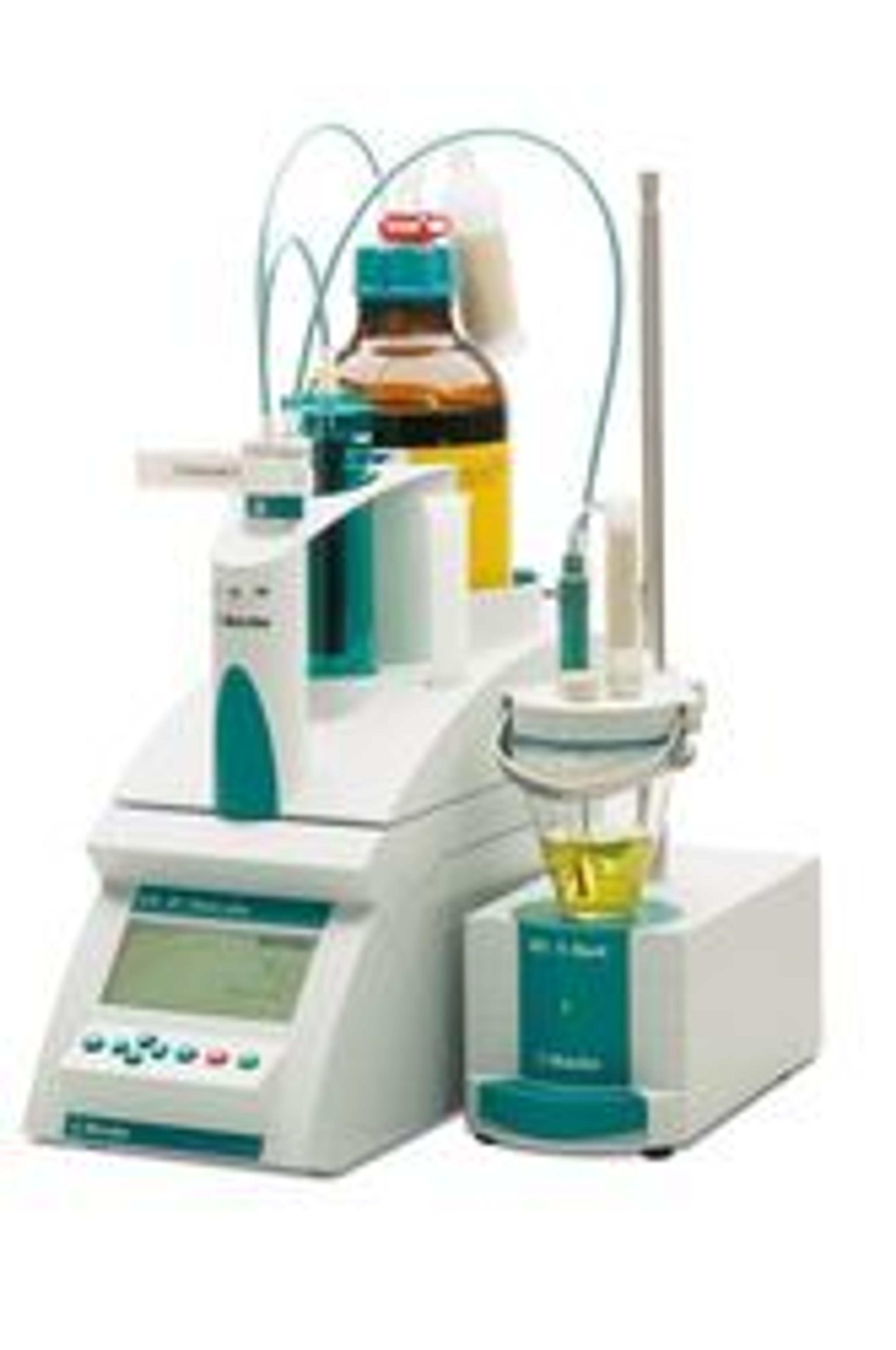
900 Touch Control – Titration was Never Easier
The 900 Touch Control features a brilliant touch screen providing for a stunning visual experience and, due to the device’s network capability, a full range of laboratory data communication possibilities is available.
High efficiency
CIP solution
New innovation for user but high price as high efficiency
Review Date: 13 Dec 2022 | Metrohm AG
Great reproducible results
Autotitration free acid
Equipment works well to produce repeatable results much better than manual titration.
Review Date: 9 Dec 2022 | Metrohm AG
The 900 Touch Control features a brilliant touch screen providing for a stunning visual experience and, due to the device’s network capability, a full range of laboratory data communication possibilities is available.
Up to 14 methods can each be linked to a favorite icon on the touchscreen. Whether one, two or more users – each one has direct access to his or her most important methods on the start screen and starts them with a single touch of the respective icon.
What used to require a PC is now possible with the 900 Touch Control due to advanced microelectronics. An Ethernet interface provides direct access to the intranet. Reports can be printed on any network printer or saved on a network storage device or an external hard disk. Moreover, results and other data can be sent directly to any LIMS or – in a stand-alone solution – saved on the spot in the system’s archive.
Further features of the 900 Touch Control:
- USB-interface for printer, keyboard or mouse. Methods and determinations can be directly saved on a memory stick and read in again from there
- PDF-generator for creating forge-proof reports
- Automatic electrode test for always reliable results
- Autostart function: coulometric Karl-Fischer titrations are started automatically once sample is added
- User dialogue in many languages including Chinese
- Compliance with FDA-regulation 21 CFR, Part 11