Diagnostic Tests/Kits Products & Reviews
Diagnostic Tests/Kits
Selected Filters:
AP 16 IF ELITE
DAS ItalyAP 16 IF ELITE is an innovative and fast processor able to run 16 IFA slides, it is equipped with a sliding-sample barcode reader and a camera for slide bar code reading.
AP BLOT ELITE
DAS ItalyAP BLOT ELITE is a fully automated Blot processor able to manage in the same run sample dilution and its dispensation, reagent dispensation, incubation times, strip washings, dot assessment by image processing of strip
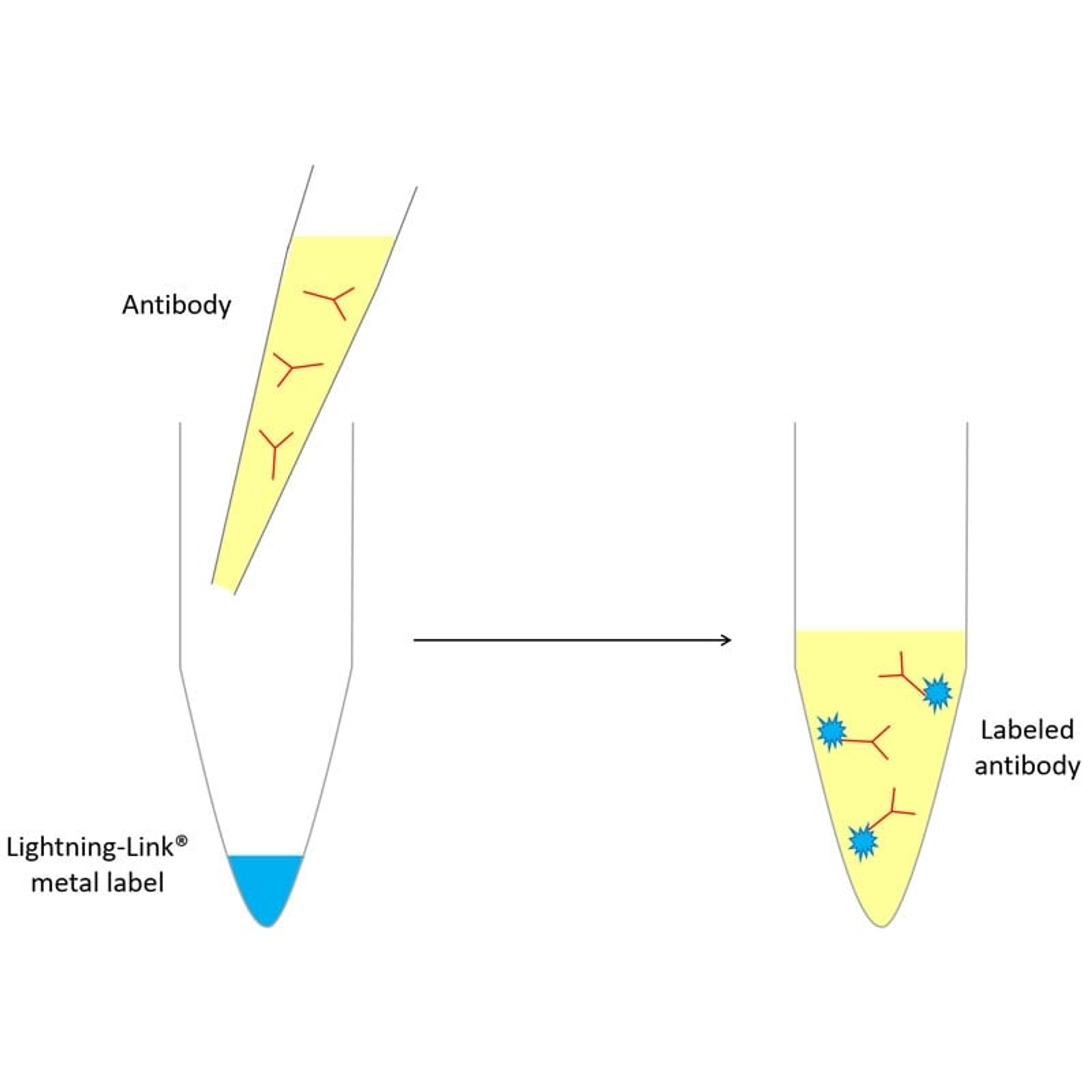
Lightning-Link Metal Labeling Kits
ExpedeonCurrent metal conjugation protocols can be complex and time consuming procedures, and may result in low antibody recovery. Lightning-Link Metal Labeling Kits allow metal / antibody labeling to be set up in less than 30 seconds – simply add the antibody solution to the activated metal ligand!
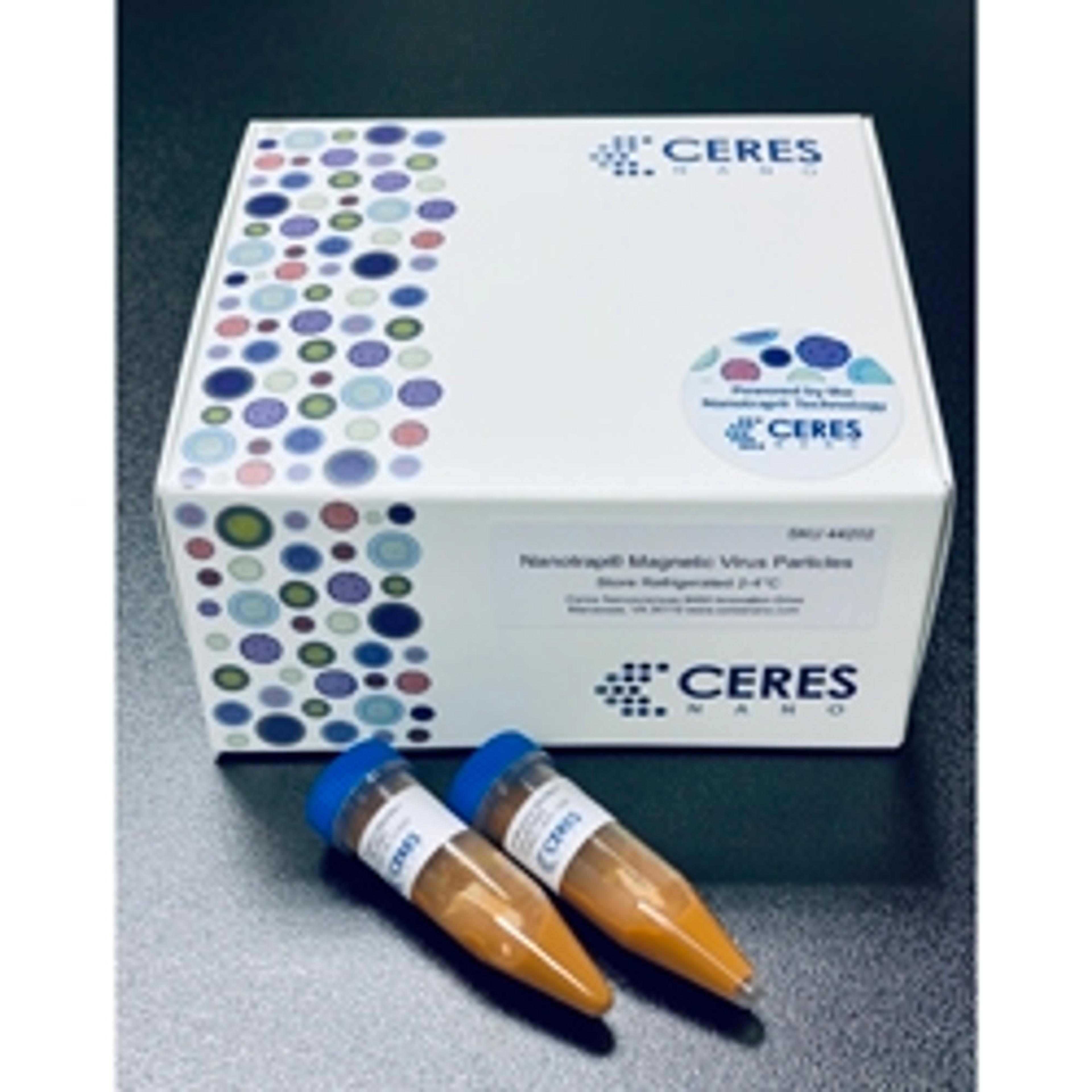
Nanotrap® Virus Capture Kit (50)
Ceres Nanosciences, Inc.The Ceres Nanosciences Nanotrap® Virus Capture Kit provide a way to concentrate viruses from complex biological matrices in order to have high quality input material for your downstream analytical methods
Platelia SARS-CoV-2 Total Ab Assay
Bio-RadPlatelia SARS-CoV-2 Total Ab Assay. Detection of IgM, IgA and IgG in One Test.
ErbaLisa COVID-19 IgG and IgM Antibody ELISA Kits
Erba MannheimCE-marked Enzyme Immunoassay (ELISA) kits for the detection of IgG and IgM antibodies to SARS-CoV-2 in human serum
COVID-19 ImmunoRank™ Neutralization MICRO-ELISA Kit
Leinco TechnologiesThe COVID-19 ImmunoRank™ Neutralization MICRO-ELISA test for detection of circulating SARS-CoV-2 neutralizing antibodies. The assay is designed to detect antibodies of all Ig classes in human plasma or serum that bind to the SARS-CoV-2 receptor binding domain (RBD) and are capable of blocking the binding of the RBD to angiotensin-converting enzyme 2 (ACE2), the viral entry receptor on the surface of target cells.
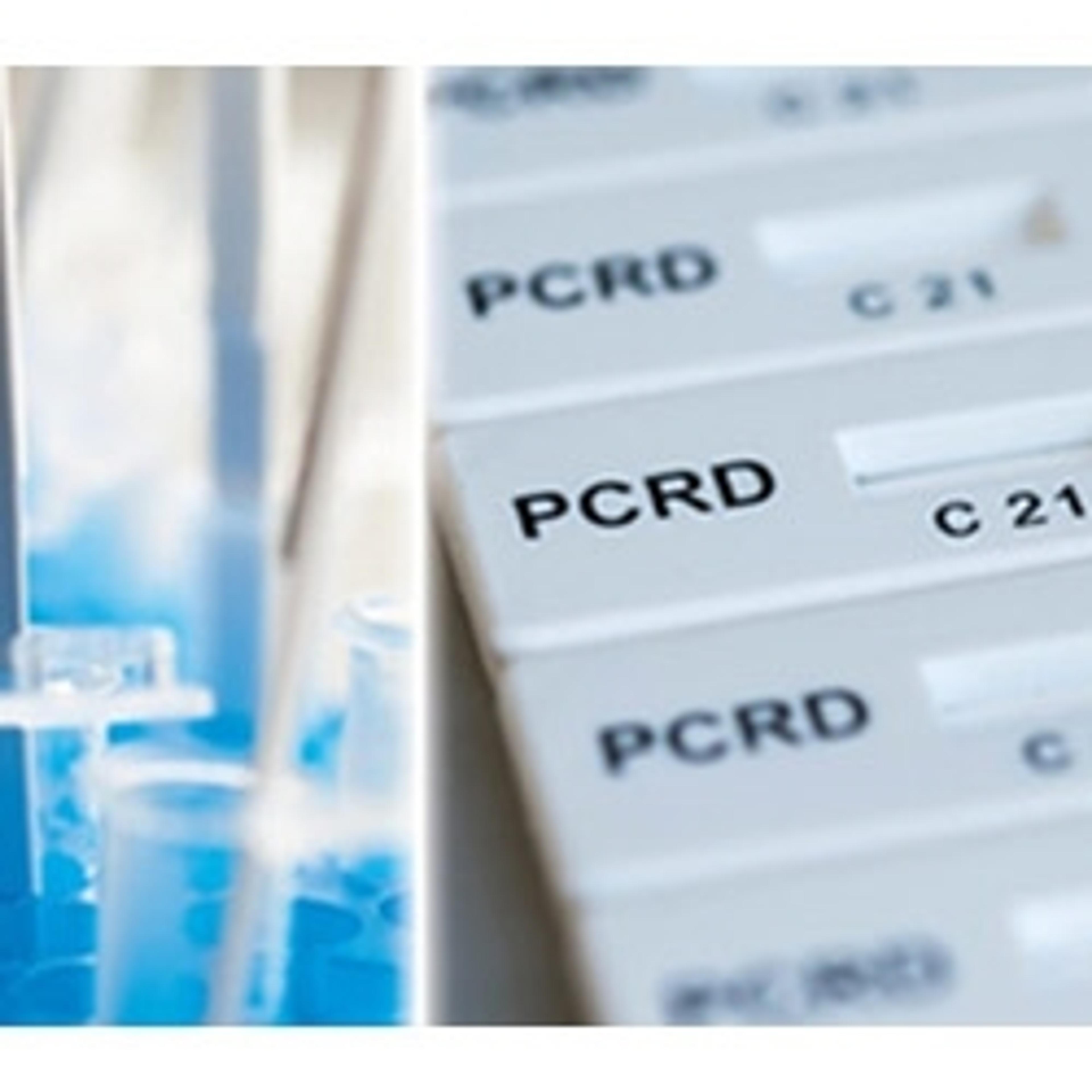
PCRD & PCRD FLEX
Abingdon HealthRapid nucleic acid detection post isothermal amplification for decentralised, point of care molecular testing
Bio-Plex Multiplex SARS-CoV-2 Serology Assay Kits and Reagents
Bio-RadProvides sensitive and specific measurements of antibodies against four SARS-CoV-2 proteins: nucleocapsid, receptor binding domain, spike 1, and spike 2 viral proteins.
Bio-Plex Multiplex SARS-CoV-2 Serology Assays
Bio-RadProvides sensitive and specific quantitation of antibodies against four SARS-CoV-2 proteins: nucleocapsid, receptor binding domain, spike 1, and spike 2 viral proteins.
BioReady Citrate Gold Nanospheres - 40 nm
nanoComposixBioReady Gold Nanospheres - Bare (Citrate) - 40 nm, 20 OD in aqueous 0.02 mM sodium citrate
BioReady Citrate Gold Nanospheres - 80 nm
nanoComposixBioReady Gold Nanospheres - Bare (Citrate) - 80 nm, 20 OD in aqueous 0.02 mM sodium citrate
BioReady Streptavidin Gold Nanospheres - 40 nm
nanoComposixBioReady Gold Nanospheres - Streptavidin - 40 nm, 10 OD in aqueous conjugation buffer
BioReady Streptavidin Gold Nanoshells - 150 nm
nanoComposixBioReady Gold Nanoshells - Streptavidin - 150 nm, 10 OD in aqueous conjugation buffer
LIAISON® SARS-CoV-2 TrimericS IgG
Diasorin LtdThe LIAISON® SARS-CoV-2 TrimericS IgG assay enables the reliable detection of Trimeric S spike protein IgG antibodies – the body’s natural defense response against SARS-CoV-2.
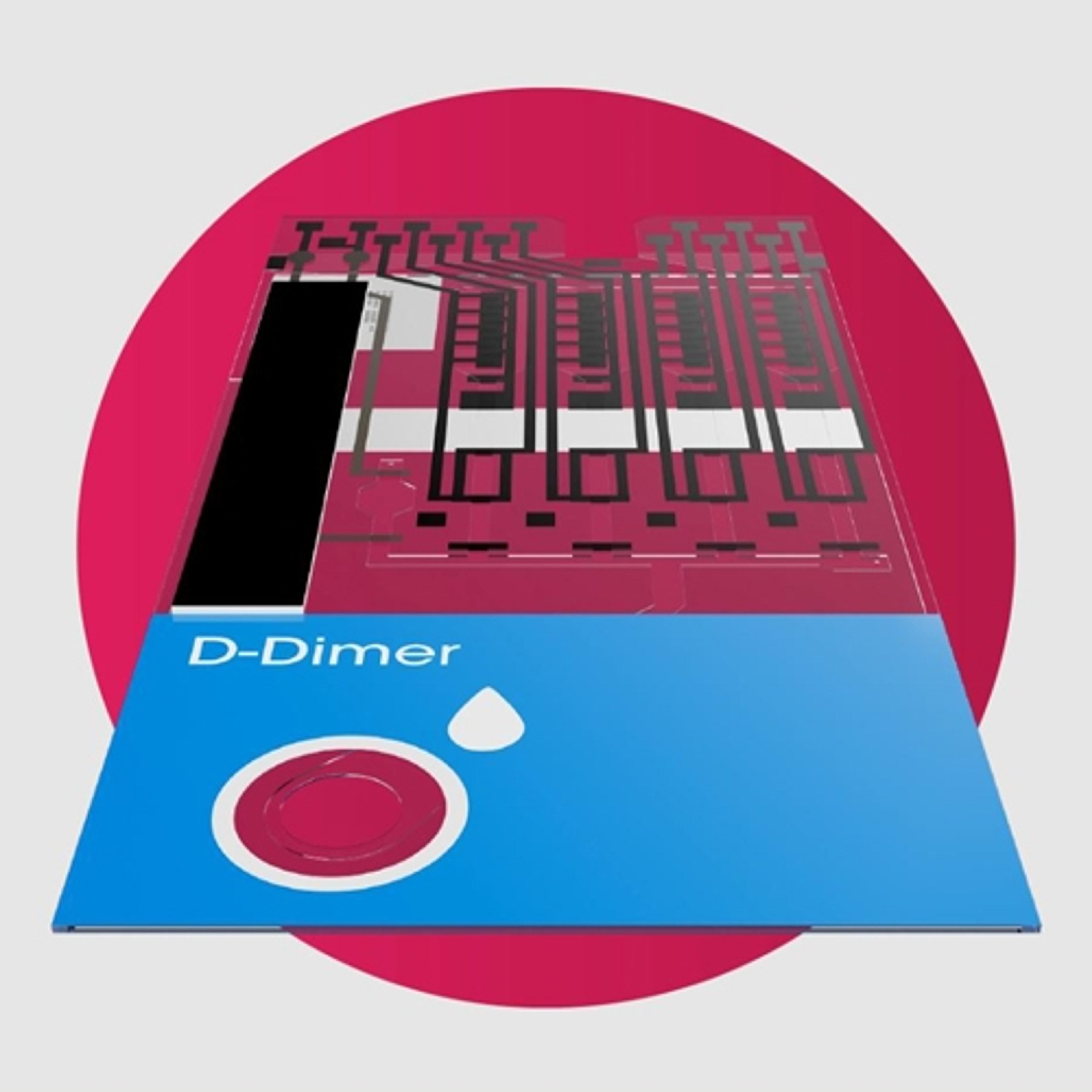
D-Dimer Test
LumiraDxThe LumiraDx D-Dimer Test is an easy to use, fast microfluidic immunoassay designed to rapidly quantify D-Dimer levels in whole blood and plasma. It is the only direct fingerstick D-Dimer assay available today*, aiding healthcare professionals to exclude deep vein thrombosis (DVT) and pulmonary embolism (PE) in symptomatic patients with confidence** - in only 6 minutes.
HbA1C Test
LumiraDxThe LumiraDx HbA1c test is an easy to use, fast microfluidic immunoassay designed to rapidly quantify HbA1c in fingerstick and venous whole blood. Used with the LumiraDx Platform, the LumiraDx HbA1c test delivers rapid, reliable results in approximately < 7 minutes at the point of care.
NT-proBNP Test
LumiraDxThe LumiraDx NT-proBNP test is an easy to use, fast microfluidic immunoassay designed to rapidly quantify NT-proBNP in human capillary and venous whole blood and plasma samples (Lithium Heparin). Used with the LumiraDx Platform, the LumiraDx NT-proBNP test delivers rapid, reliable results in 12 minutes at the point of care.
SARS-CoV-2 Ab Test
LumiraDxThe LumiraDx SARS-CoV-2 Ab Test is a microfluidic immunofluorescence assay for qualitative detection of total antibodies to SARS-CoV-2 in human whole blood (capillary fingerstick or venous), plasma or serum for indication of recent or prior infection. Used with the LumiraDx Platform the Test delivers rapid results at the point of care.
SARS-CoV-2 Ag Test
LumiraDxThe LumiraDx SARS-CoV-2 Ag Test is a microfluidic immunofluorescence assay for the direct and qualitative detection of nucleocapsid protein antigen in nasal and nasopharyngeal swab specimens from individuals suspected of COVID-19 or asymptomatic individuals. Used with the LumiraDx Platform the test delivers rapid results at the point of care.
Sars-CoV-2 Ag Ultra Test
LumiraDxLab-comparable results in just 5 minutes - With excellent performance and results in just 5 minutes, the LumiraDx SARS-CoV-2 Ag Ultra test enables you to accurately and confidently test more patients, optimize clinic workflows and help triage patients without delay
SARS-CoV-2 Ag Pool Test
LumiraDxThe LumiraDx SARS-CoV-2 Ag Pool Test is a rapid microfluidic immunofluorescence assay for the qualitative detection of the nucleocapsid protein antigen in nasal or nasopharyngeal swab specimens pooled from up to 5 individuals suspected of COVID-19 or up to 5 asymptomatic individuals. Used with the LumiraDx Platform the test offers a rapid, scalable and cost-effective screening solution for infectious individuals.