OMNIS Client/Server
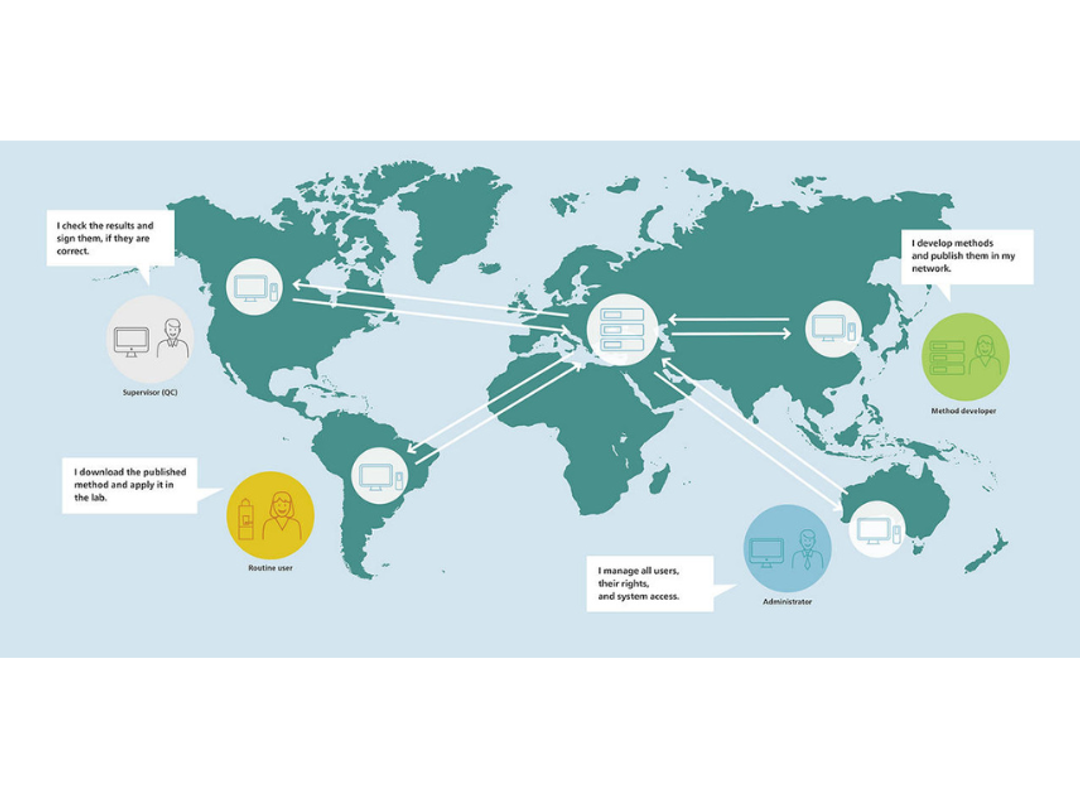
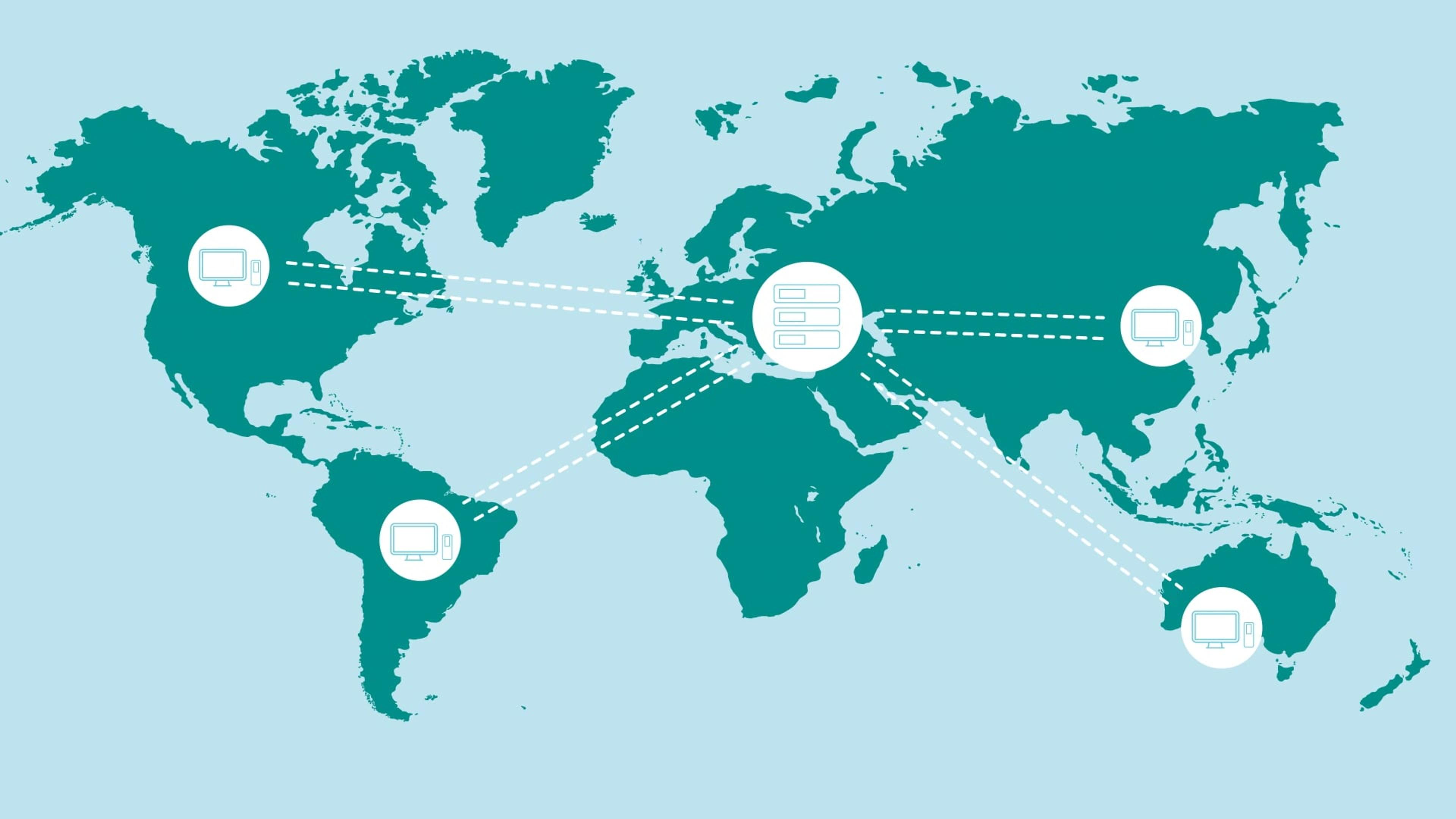
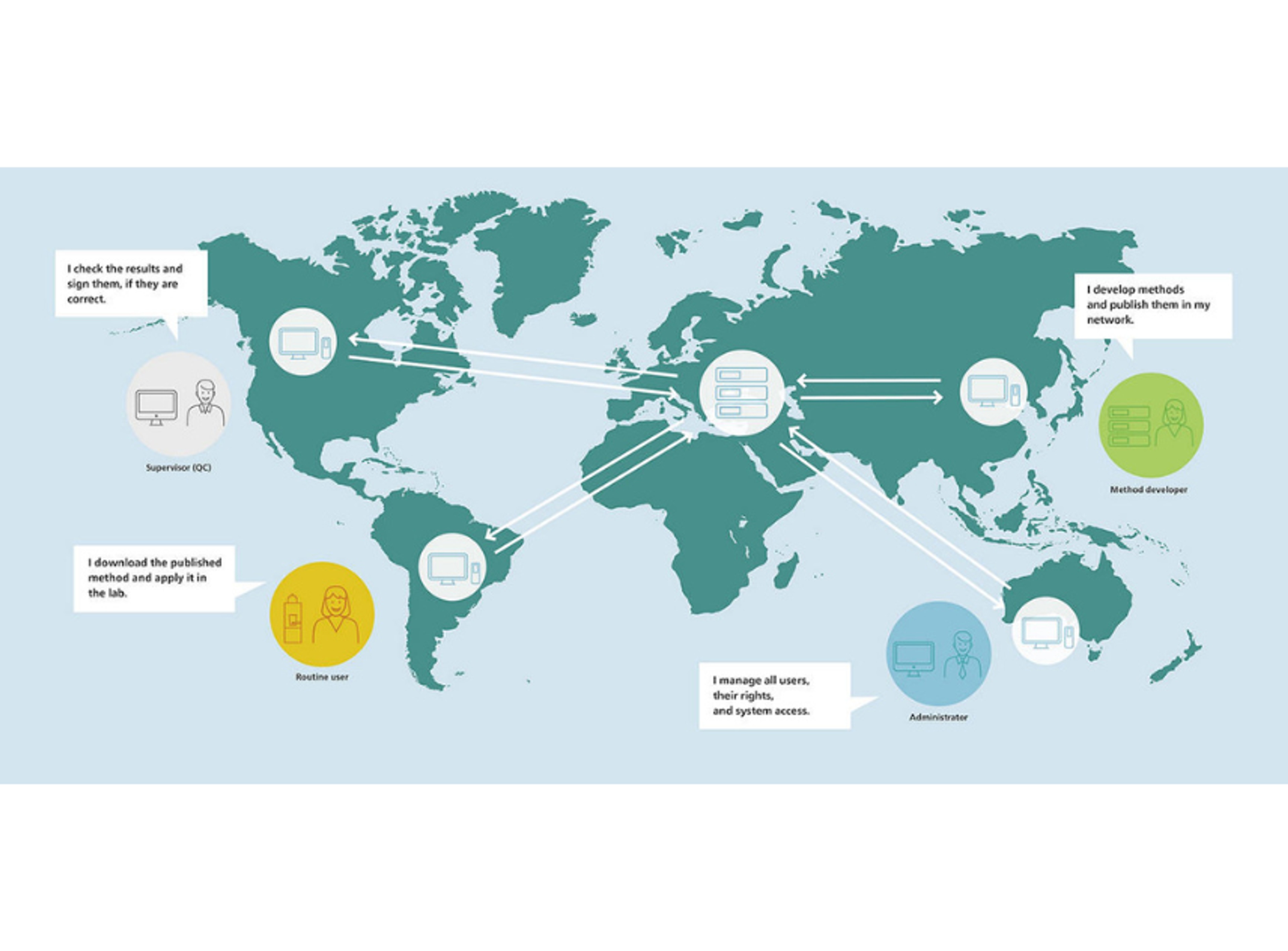
OMNIS Client/Server optimizes lab collaboration with centralized data and user management, supporting up to 500 clients. It ensures secure data sharing, configurable roles, and traceability compliant with regulations like FDA 21 CFR Part 11. Robust even during server downtime, it allows work for up to 48 hours uninterrupted.
Can meet various experimental needs/ 可满足各种实验需求。
Determination of acid concentration and metal content in chemical production raw materials, process products and finished products/化工生产产品原料、过程产品、成品中酸浓度及金属含量的测定
This equipment can use different methods to analyze and test, such as acid-base titration, precipitation titration, complex titration, redox titration, etc. There are also many modes, DET, KFT, MET, etc. After setting the method, the operation is simple and easy to use. Equipment can improve the accuracy of experimental data and improve the work efficiency of analysts. 此设备可以使用不同方法来分析化验,比如酸碱滴定、沉淀滴定、络合滴定、氧化还原滴定等,模式也有多种、DET、KFT、MET、等,设定方法后操作简单易上手,此设备可以提高实验数据的精确度,提高分析人员的工作效率
Review Date: 2 Dec 2024 | Metrohm AG
OMNIS Client/Server platform transforms how laboratories manage data and collaborate, delivering flexibility, security, and compliance in a single, scalable solution. Whether for a small team or a large network, it adapts to your needs and ensures smooth operations at every level.
Key Features
- Centralized Data Management
- Manage all your laboratory data, instruments, and standard operating procedures (SOPs) from one central platform.
- User roles and rights can be configured easily to match team member responsibilities.
- Network Collaboration
- Supports up to 500 clients in a single network, enabling seamless sharing of data and methods across teams.
- Any user in the network can access results or analyses created by others with just one click, regardless of location.
- Flexible and Scalable Design
- Expand your system as your lab grows by adding more clients or integrating additional instruments.
- Compatible with Metrohm Titrando systems and devices using RS-232 or Ethernet interfaces, protecting your current investments.
- Customizable User Experience
- Each user can configure their OMNIS interface to match their preferences and role-specific needs.
- Available in multiple languages, ensuring usability for diverse teams.
- Robust Offline Functionality
- In case of server connectivity loss, users can continue working with full functionality for up to 48 hours.
- All activities during downtime are saved locally and synced with the server once reconnected, ensuring no data loss.
- Secure Data Handling
- Meets high standards for data security and traceability, with all client-server communications encrypted using TLS.
- Full compliance with FDA 21 CFR Part 11 and EudraLex Volume 4, Annex 11, ensuring reliable audit trails and regulatory adherence.
- Simplified Compliance Management
- Features dedicated modes (productive, qualification, and service) to separate service data from routine lab data.
- Central audit trails provide detailed records of actions, changes, and user activities for effortless compliance.
- Long-Term Stability
- An optional Long-Term Support (LTS) version ensures stable, reliable system performance for up to five years.