Drug discovery > Drug Manufacturing Products & Reviews
Products, services, reviews and techniques used in pharmaceutical product scale-up, formulation, shipping and manufacturing processes.
Selected Filters:
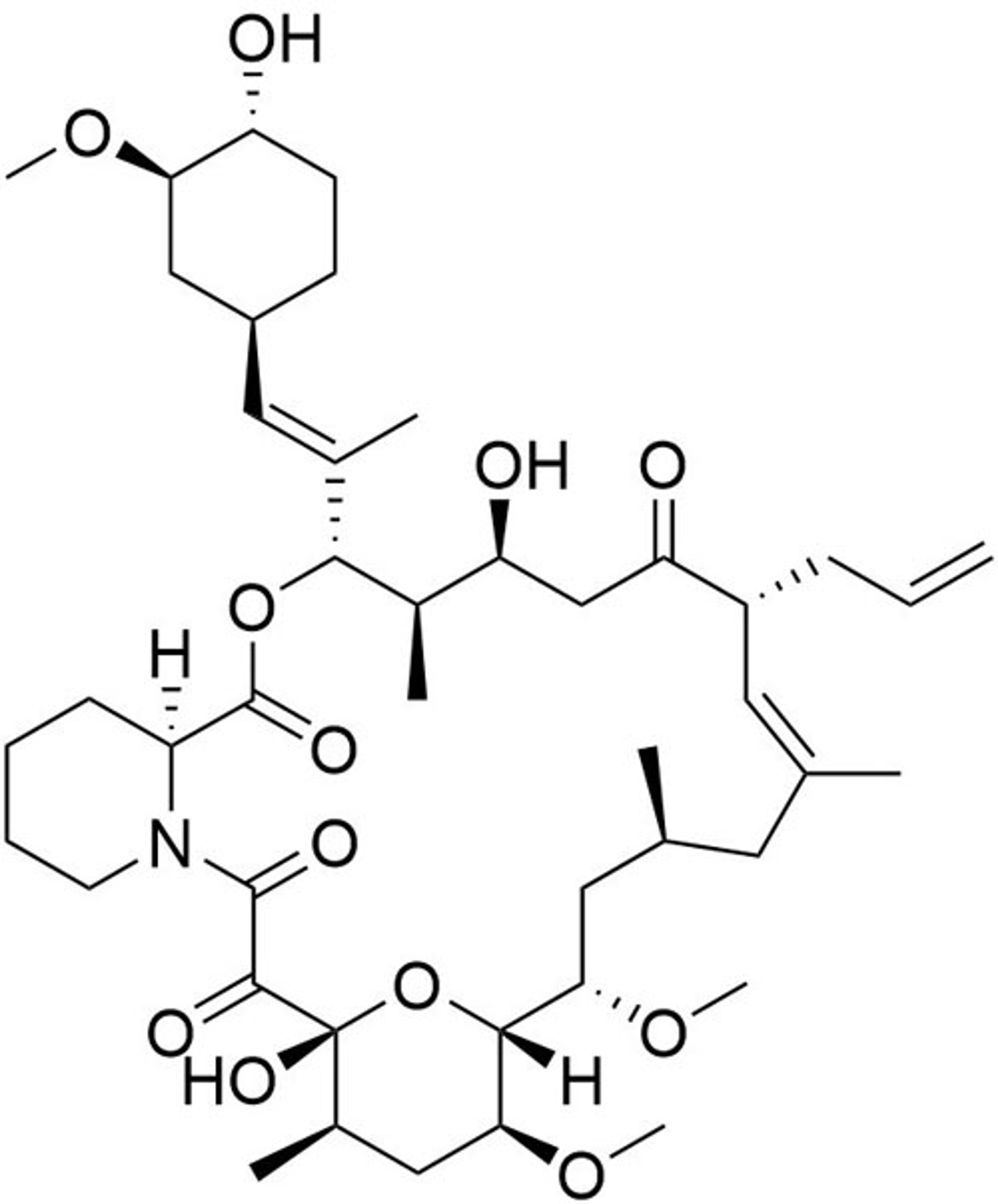
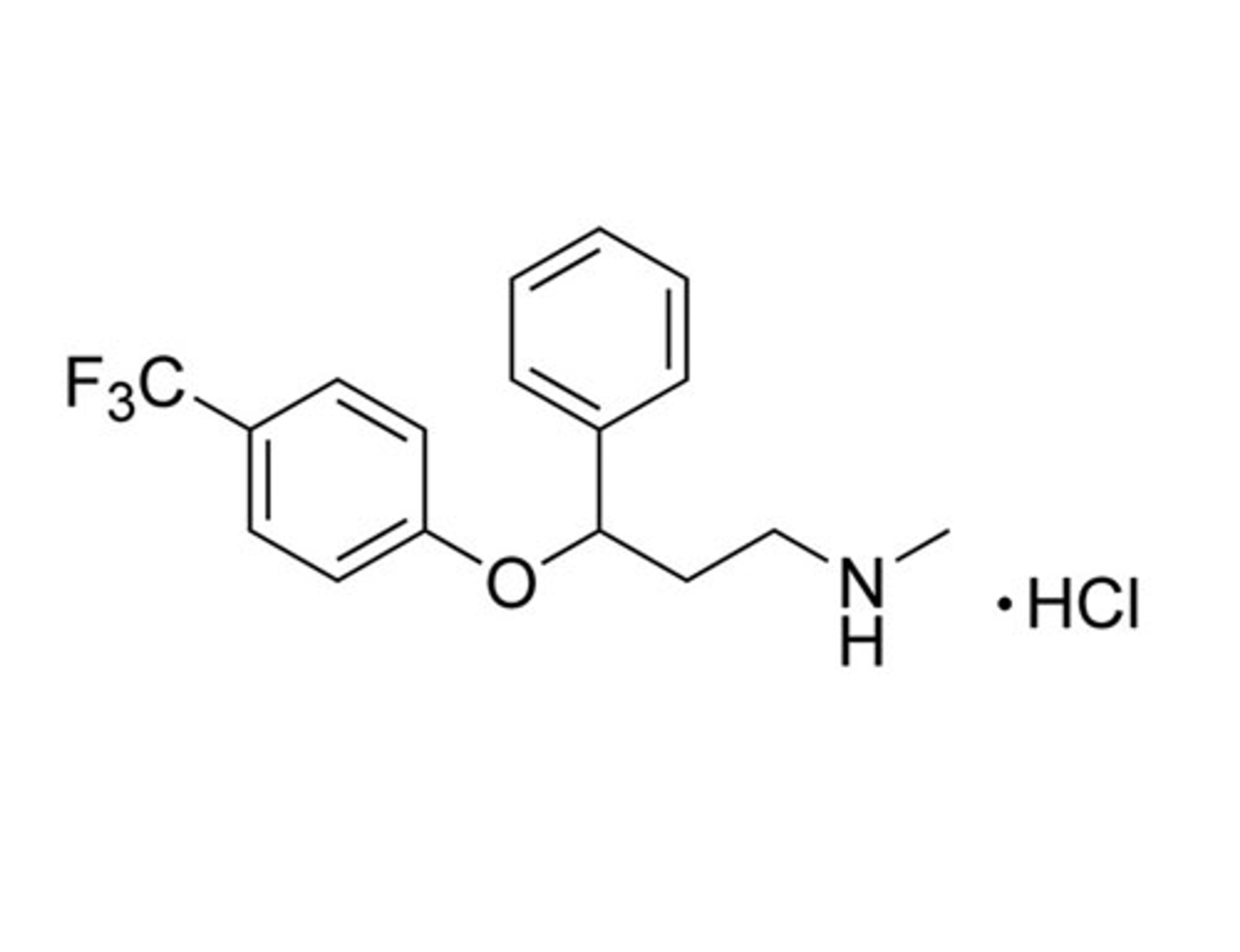
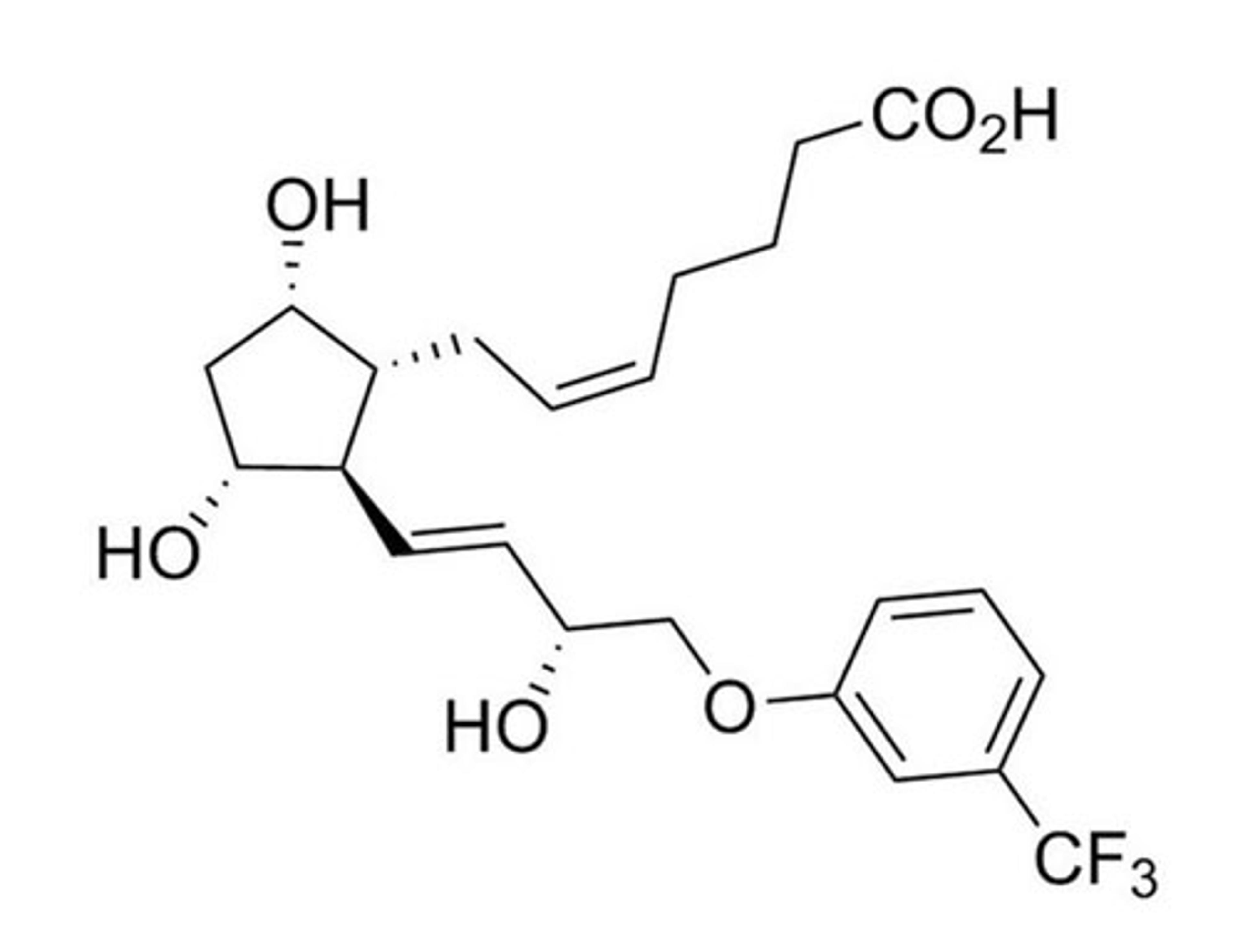
Fluprostenol
STEMCELL Technologies Inc.Prostanoid pathway activator; Activates prostaglandin F2a receptor
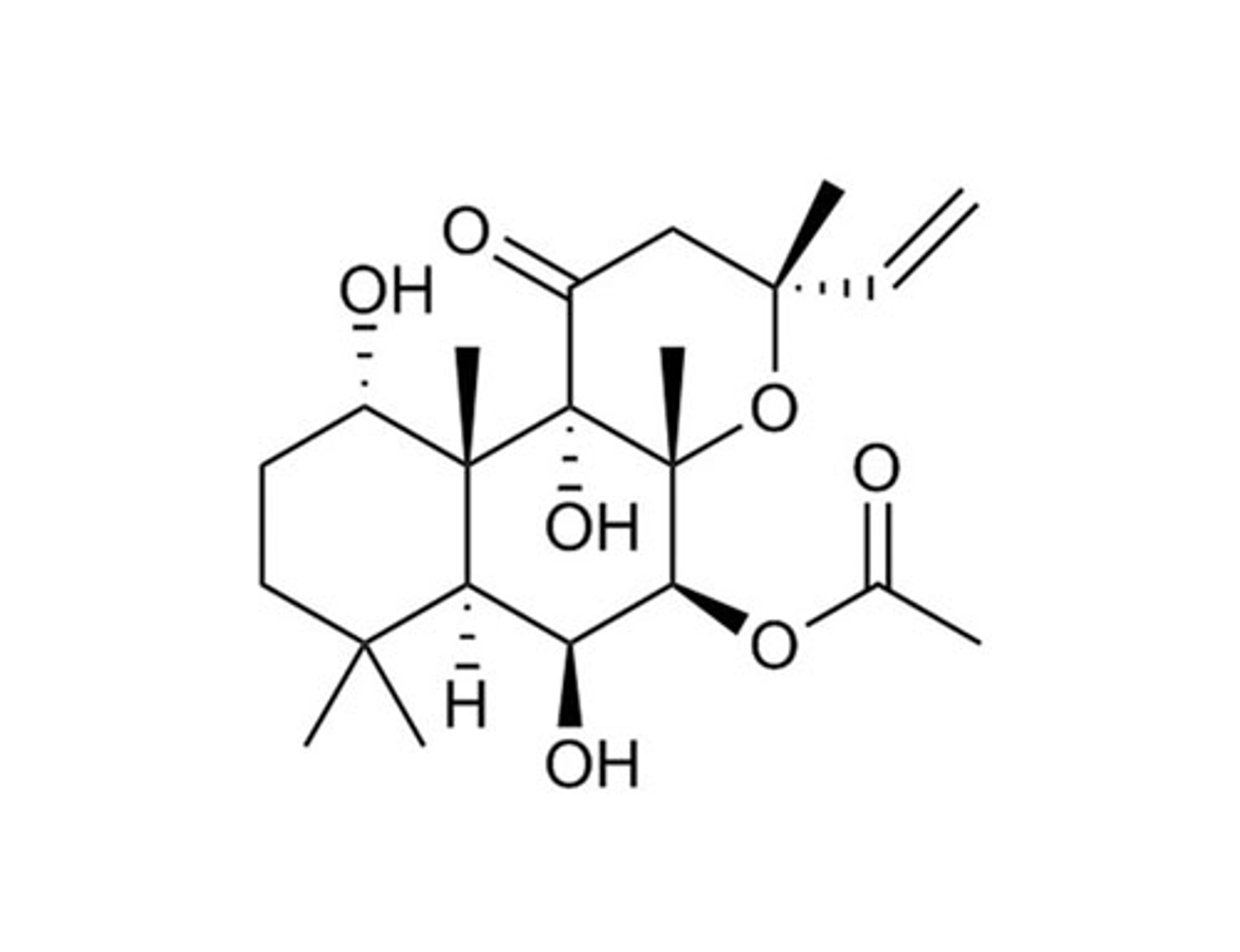
Fumonisin B1
STEMCELL Technologies Inc.Inhibitor of sphingolipid synthesis and protein serine/threonine phosphatases
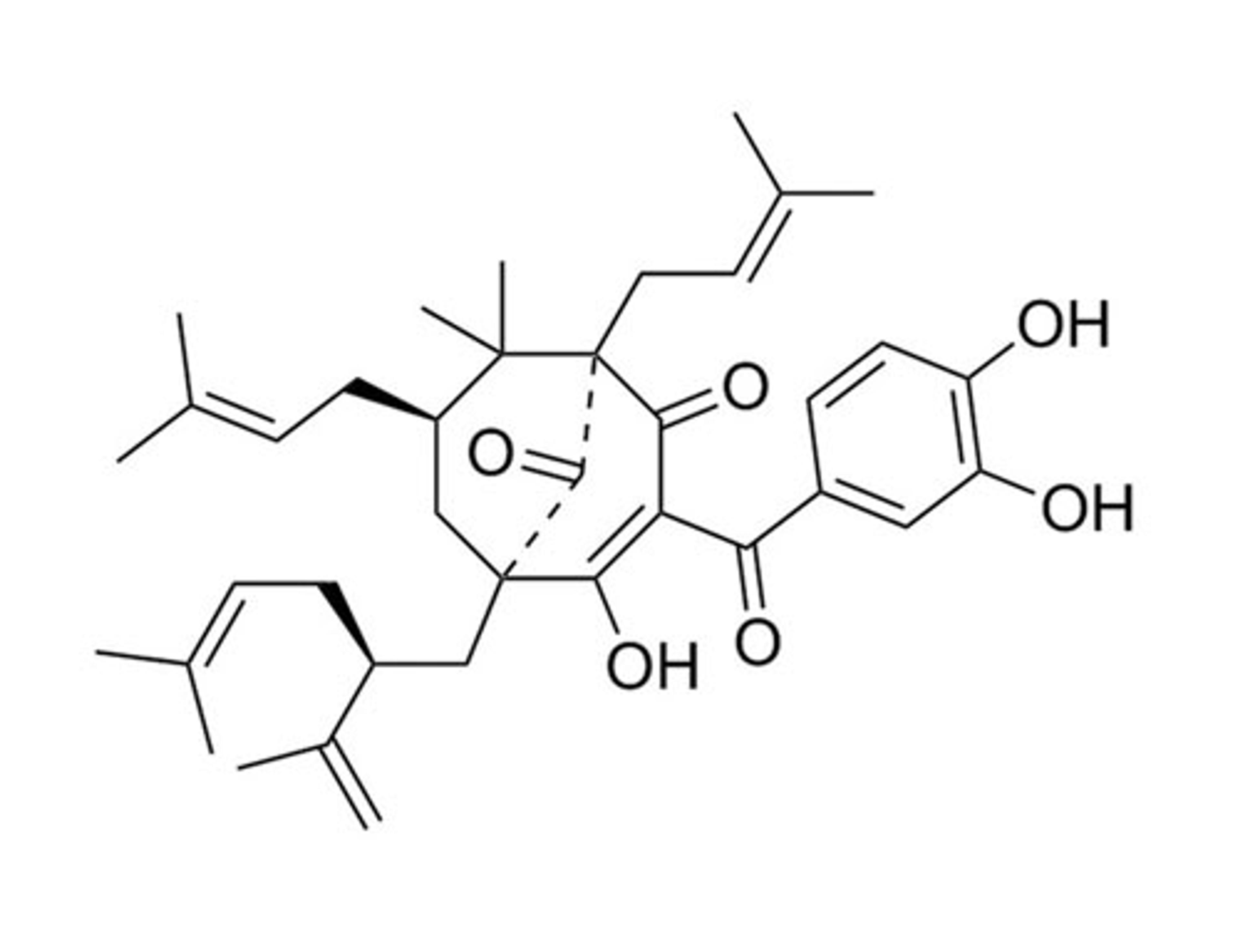
Garcinol
STEMCELL Technologies Inc.Epigenetic modifier; Inhibits histone acetyltransferases (HATs) p300 and pCAF
EasySep™ Human Naïve B Cell Isolation Kit
STEMCELL Technologies Inc.9-Minute cell isolation kit using immunomagnetic negative selection
EasySep™ Human Naïve CD4+ T Cell Isolation Kit
STEMCELL Technologies Inc.Immunomagnetic negative selection kit
EasySep™ Human Naïve CD4+ T Cell Isolation Kit II
STEMCELL Technologies Inc.Immunomagnetic negative selection cell isolation kit
EasySep™ Human Naïve CD8+ T Cell Enrichment Kit
STEMCELL Technologies Inc.Immunomagnetic negative selection kit
EasySep™ Human Naïve CD8+ T Cell Isolation Kit
STEMCELL Technologies Inc.Immunomagnetic negative selection kit
EasySep™ Human Naïve CD8+ T Cell Isolation Kit II
STEMCELL Technologies Inc.Immunomagnetic negative selection kit
EasySep™ Human Naïve Pan T Cell Isolation Kit
STEMCELL Technologies Inc.Immunomagnetic negative selection kit
EasySep™ Human Neutrophil Isolation Kit
STEMCELL Technologies Inc.14-Minute cell isolation using immunomagnetic negative selection
EasySep™ Human NK Cell Enrichment Kit
STEMCELL Technologies Inc.Immunomagnetic negative selection cell isolation kit
EasySep™ Human NK Cell Isolation Kit
STEMCELL Technologies Inc.8-Minute cell isolation kit using immunomagnetic negative selection
EasySep™ Human Pan-B Cell Enrichment Kit
STEMCELL Technologies Inc.Immunomagnetic negative selection cell isolation kit
EasySep™ Human Pan-CD25 Positive Selection and Depletion Kit
STEMCELL Technologies Inc.Immunomagnetic positive selection cell isolation kit