Bioprocessing Salt Active Nucleases - Physiological Conditions
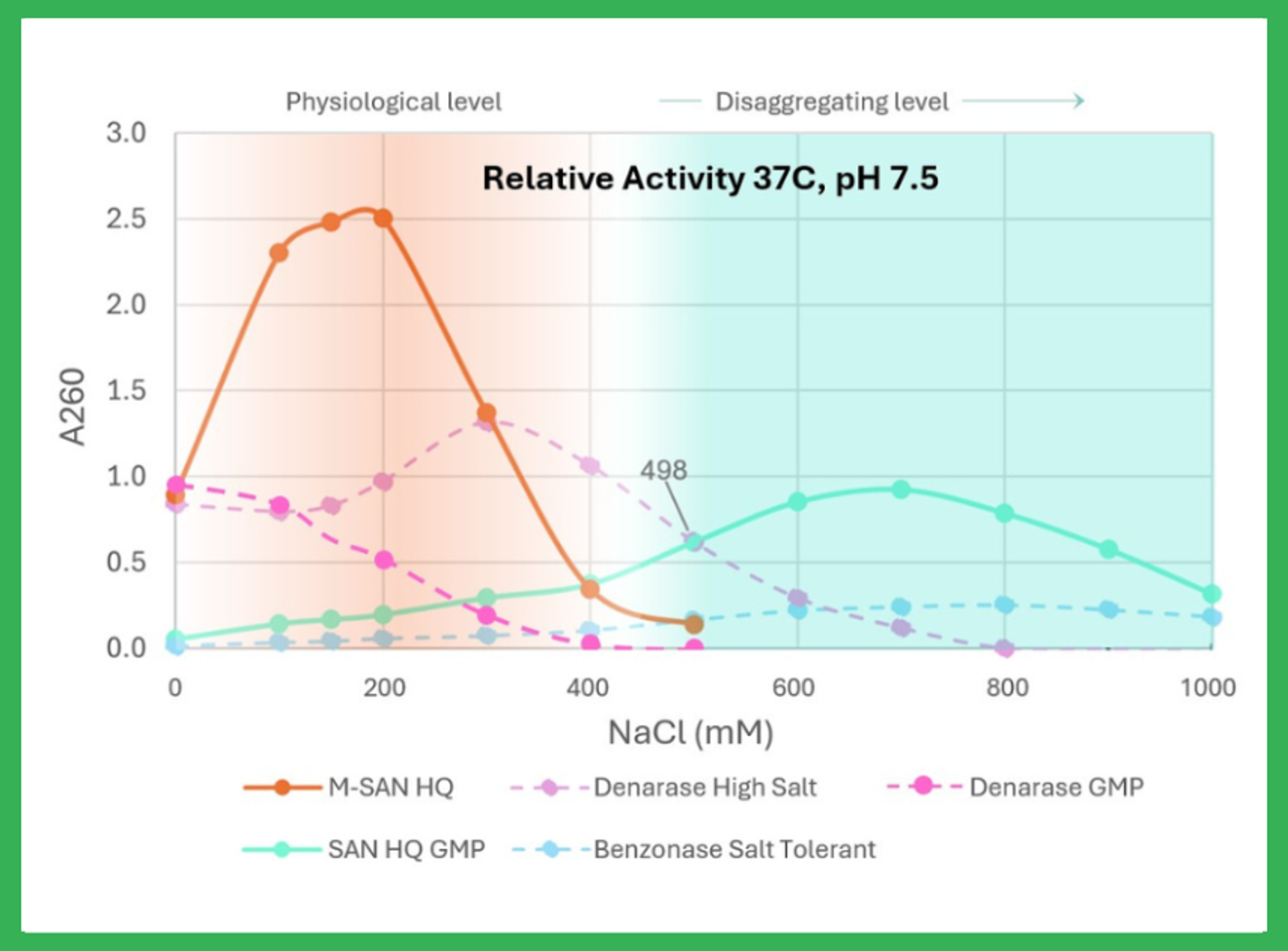
Nonspecific endonuclease is highly active at physiological conditions (125–200 mM) for DNA removal during protein production, vaccine manufacturing, and viral vector preparation. M-SAN High Quality can be used directly in cell media, which improves efficiency and yield. Designed to replace Benzonase without workflow changes. M-SAN HQ excels in ensuring higher purity and efficiency. High purity (≥ 99%). Compatible with ELISA ki…
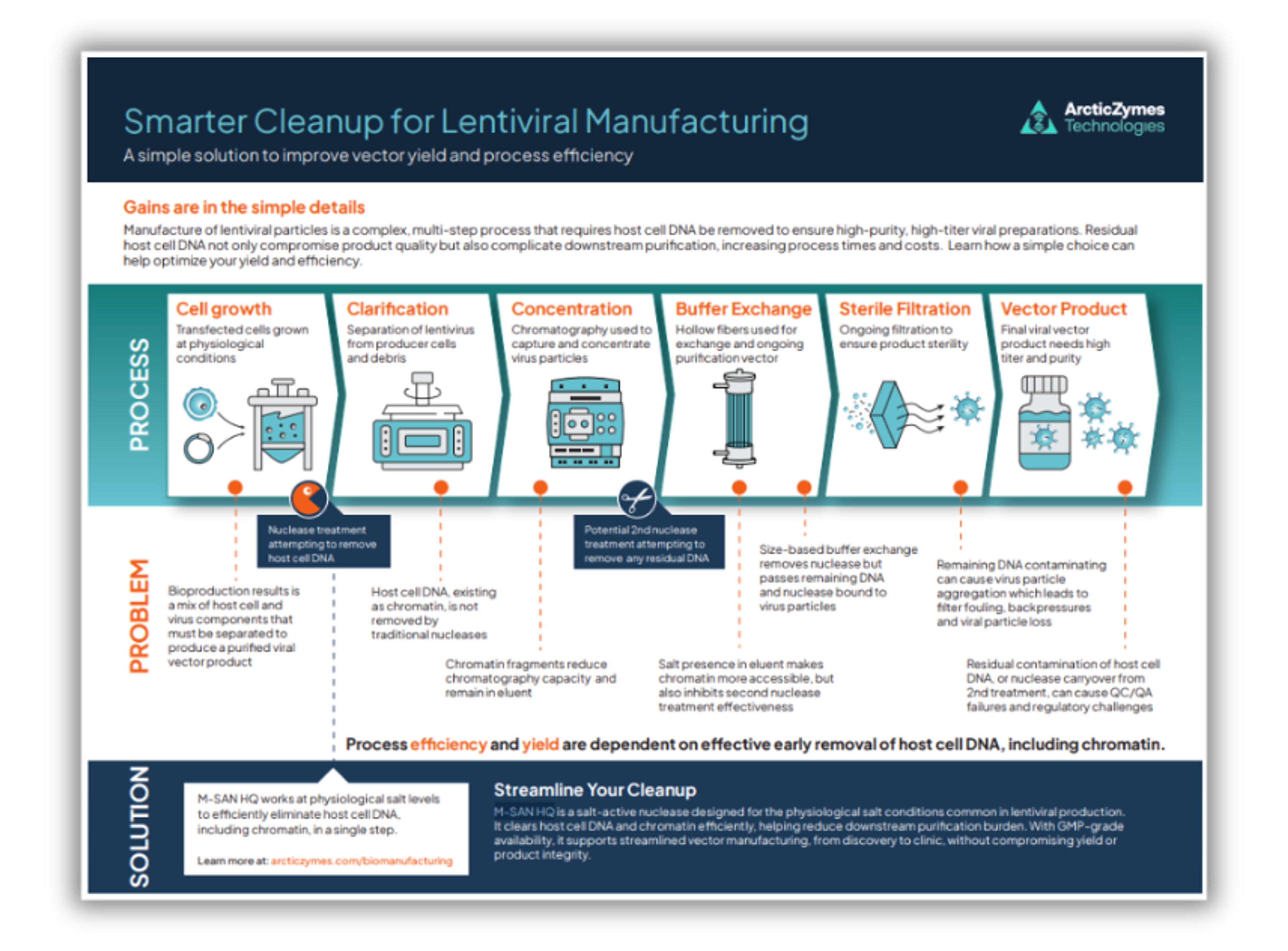
This novel, nonspecific endonuclease is active over a broad pH range and displays optimum activity at salt concentrations between 125 – 250 mM. Due to its excellent performance at physiological conditions, M-SAN HQ can be used directly in the cell medium or the harvested supernatant without buffer adjustments. This makes M-SAN HQ ideal for the manufacturing of fragile vectors such as lentiviruses and retroviruses.
M-SAN HQ can be directly used in medium without buffer adjustments The high activity of M-SAN HQ at standard cell medium conditions leads to improved DNA clearance compared to other commonly used nucleases. In the data, an over 2-fold reduction in residual DNA was achieved.
Key Benefits
- Compatibility: Ideal for working with both fragile and robust viral vectors and proteins in a variety of cell media
- Optimum activity: Optimization for cell media salinity allows both shorter DNA fragments and reduced incubation times.
- Cost-Effectiveness: Reduced need for additional reagents and steps can lower production costs
- Quality: Maintains the integrity of sensitive or labile biological molecules, ensuring a higher quality end product
- Flexibility: Can be used directly in a variety of media without the need for customization
Key Features - High purity (≥ 99%)
- No protease detected
- Endotoxin-tested
- Animal origin-free production
- Supplied with extended product documentation
- ELISA Kit available