Search results for "Rigaku Analytical Devices"
25
Selected Filters:
Editorial Article
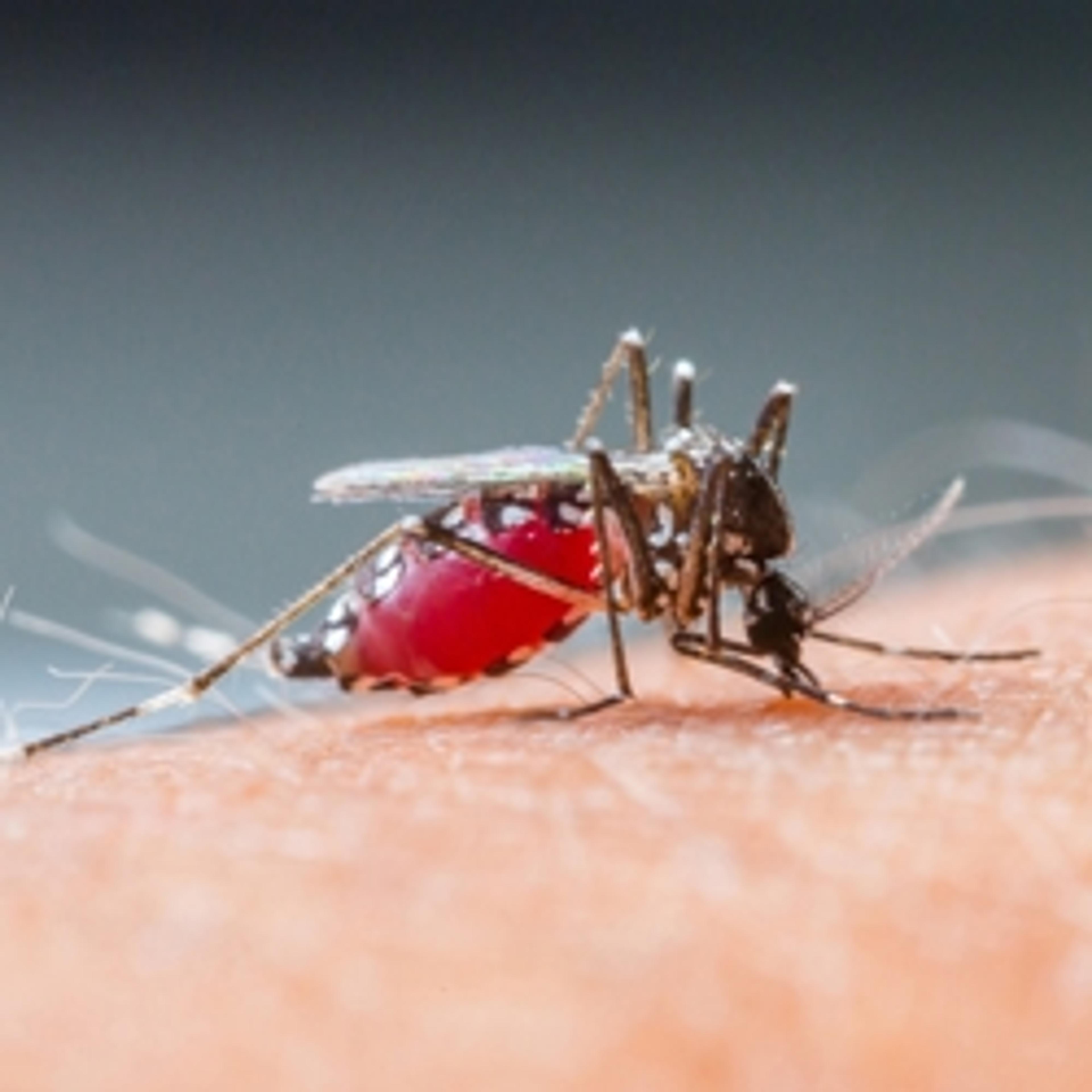
Keeping Sports Clean: Unique Chemical Fingerprinting of Cheaters
State-of-the-art mass spectrometry helps scientists improve accuracy and keep ahead of doping trends
Product News
Microscan Introduces MicroHAWK Barcode Readers
Industry News
Huge Chinese Investment in ‘Silicon Valley’ Rival Set to Accelerate Semiconductor Innovation
Liverpool Accelerator Science Symposium considers growing importance and economic potential of particle colliders
Expert Insights
Faster accessibility and more intuitive FTIR spectroscopy
Watch this webinar to discover the innovation taking place within the FTIR spectroscopy field, leading to novel capabilities for a wide variety of industry applications
Product News
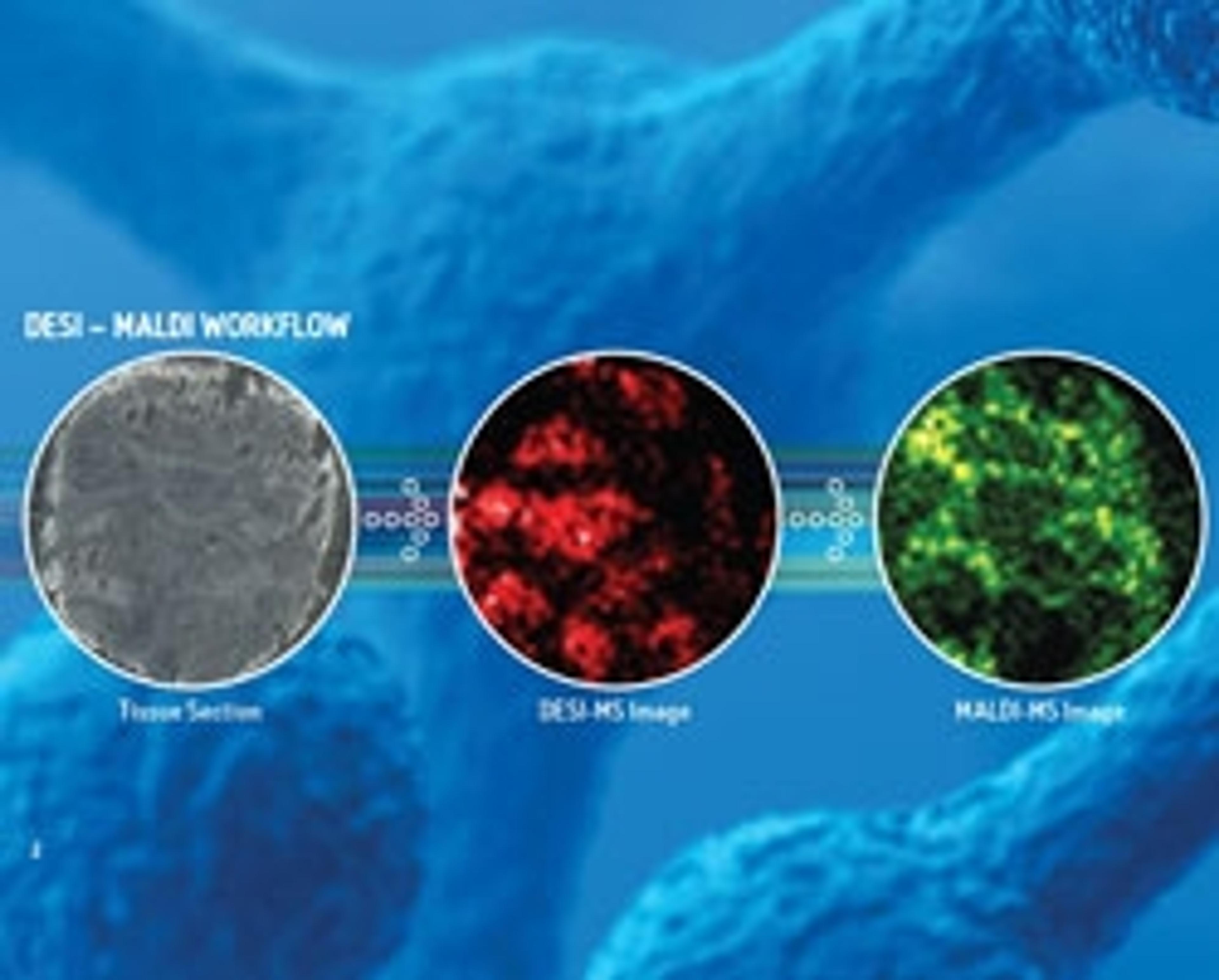
Waters Presents Advanced Technologies for Cancer Metabolism Researchers at AACR
Booth #3307
Product News